Abstract
Objective
Suspicious incidental gastrointestinal FDG uptake during positron-emission tomography/computed tomography (PET/CT) examinations can be caused by different diseases, including malignancies. However, differentiation with PET alone is difficult. The aim of this study was to investigate the potential of PET alone, contrast-enhanced CT (ceCT), and low-dose CT (ldCT) in routine PET/CT protocols for differentiation of incidental gastrointestinal lesions.
Materials and Methods
Sixty patients with incidental gastrointestinal lesions who underwent a routine PET/CT protocol with ldCT and ceCT were retrospectively analysed. The PET lesions were evaluated regarding their FDG uptake patterns and the standard uptake value. The anatomical correlates in both CT protocols were compared in regard to the correct lesion classification with the reference standard endoscopy.
Results
Sixty-two lesions were found in 60 patients (17 malignant, 10 premalignant, 5 benign, 13 inflammatory, 17 physiological). The differentiation of the FDG uptake patterns did not enable reliable lesion classification. The positive predictive value for pathology was 0.81 for ceCT in PET/CT and 0.70 for ldCT. Malignancies were detected in 100% of the patients by ceCT vs. 29.4% by ldCT. The false negative rate of ceCT for all pathologies was 31.1%, vs. 68.9% for ldCT. False positive results (17/62) could not be excluded sufficiently by either CT protocol.
Conclusion
PET/ceCT protocols provide additional benefit especially in detecting gastrointestinal malignancies as a cause of suspicious incidental gastrointestinal FDG uptake. However, since follow-up endoscopy cannot be forgone due to the considerable false negative rate even with ceCT, the addition of ceCT to a routine PET/ldCT protocol cannot be recommended for this purpose.
Positron-emission tomography/computer tomography (PET/CT) has become an important diagnostic tool for the diagnosis and treatment monitoring of many malignant diseases due to its ability to distinguish morphological abnormalities and metabolic changes in a single whole-body examination.
In about 1.3-3% of all patients who undergo PET/CT examinations with 2-deoxy-[18F] fluoro-2-D-glucose (18F-FDG) for different clinical reasons, incidental FDG uptake in the gastrointestinal tract is detected (1, 2). Previous studies have evaluated the significance of these findings, and found malignant and premalignant lesions, benign masses, and inflammatory diseases as underlying causes. However, these studies also demonstrated false-positive findings in 16-33% of the cases, which was mainly due to physiological FDG accumulation (1-3). The mechanisms of physiological gastrointestinal FDG uptake are not yet completely clear. Different reasons like foci of lymphoid tissue in the bowel, secretion of FDG by gastrointestinal cells, muscle activity, and swallowed secretions are discussed (4-7).
The differentiation between clinically relevant and irrelevant incidental gastrointestinal FDG uptake remains difficult. Since early diagnosis is fundamental in many pathological lesions (e.g., asymptomatic malignancies, premalignant lesions, and early stages of inflammatory bowel diseases), further examination, preferably by endoscopy, is strongly recommended in several studies (8-10).
In order to avoid dispensable invasive diagnostic procedures, especially in the case of physiological FDG accumulation, several attempts have been made to differentiate the underlying cause of incidental FDG uptake on the basis of PET/CT results. Tatlidil et al. (8) found that a diffuse FDG uptake pattern often correlated with physiologic mechanisms, segmental uptake with inflammation, and focal uptake with benign or malignant masses.
Furthermore, recent studies with unenhanced low-dose CT or intermediate-dose CT primarily performed for attenuation correction have also shown that abnormal findings in the CT component of PET/CT can contribute to the identification of the underlying pathophysiology of incidental gastrointestinal FDG uptake (2, 11). However, an optimized diagnostic CT protocol with the application of intravenous contrast-enhanced CT might improve the classification of suspicious incidental bowel uptake (12).
To further elaborate this issue, the aim of this study was to retrospectively compare the capability of PET alone, low-dose CT (ldCT), and contrast-enhanced CT (ceCT) for further differentiation of suspicious incidental gastrointestinal FDG uptake in patients with a routine PET/CT protocol including ldCT and ceCT. Special attention was drawn to the usefulness of CT in revealing false positive FDG uptake to prevent unnecessary invasive endoscopy.
The study was approved by the institutional review board. All patients gave their written informed consent. 5045 18F-FDG-PET/CT scans performed in our department between January 2005 and May 2011, were reviewed retrospectively for patient examinations with suspicious incidental FDG uptake in the gastrointestinal tract. Only patients with gastrointestinal FDG uptake in atypical localisation and atypical appearance without any clinical symptoms or known bowel disease in the same segment of the gastrointestinal tract were included. FDG uptake was defined as suspicious if it was focal, segmental, or diffuse over large areas of the corresponding part of the bowels (e.g., the whole colon) and with a maximum standard uptake value (SUVmax) above the background activity of the liver. Cases with slightly diffuse or multifocal FDG accumulation, especially in the colonic lumen and without bowel-wall involvement often seen in areas of fecal stasis like the caecum, are usually physiological and were excluded. Different lesions in the same patient were counted separately.
Of the 282 PET/CT patients with reported suspicious incidental gastrointestinal FDG uptake, 168 patients were examined using a routine PET/CT protocol consisting of ldCT for attenuation correction and ceCT for diagnostic purposes according to the routine workflow for patients without previous CT. 114 patients with routine single-phase protocols were not included in the study, as they were not appropriate for the direct comparison of ldCT and ceCT protocols. 60 of the 168 patients were subjected to follow-up in-house endoscopy or surgery, and were therefore available for further study according to the ethical designations. These patients were finally included in the study (mean age: 65 years, range: 41-85 years, 23 female, 37 male). The primary diseases of the patients leading to the PET/CT referral were lung cancer (n = 11), melanoma (n = 7), esophageal cancer (n = 3), gastric cancer (n = 3), hematological disease (n = 4), head and neck cancer (n = 12), rectosigmoid cancer (n = 2), osteosarcoma (n = 1), endometrial cancer (n = 1), hepatocellular carcinoma (n = 1), cancer of unknown primary (n = 8), fever of unknown origin (n = 5), and autoinflammatory diseases (n = 3).
All patients fasted for at least 6 hours before the PET/CT study, and had blood glucose levels of 3.3-8.5 mmol/L. 18F-FDG was injected at a dose of 320-495 MBq (mean: 398 MBq) followed by an uptake time of 50-97 minutes (mean: 62 minutes). 1000 mL of Mannitol (2.5%) was administered to all patients as a negative oral CT contrast agent.
The PET/CT scans were performed as a whole-body examination from the base of the skull to the upper thigh with Hi-Rez Biograph 16 (Siemens Health Care, Knoxville, TN, USA), consisting of a three-dimensional LSO-PET and a 16-row multi-detector CT (peak voltage: 120 kVp, tube current: 120-250 mAs, rotation time: 0.5 seconds, collimation: 0.75 mm in the thorax and 1.5 mm in the abdomen, table feed: 12/24 mm). Initially, a non-enhanced low-dose CT (120 kV/30 mAs) was acquired for attenuation correction, followed by a contrast-enhanced CT (arterial phase for neck and thorax and portovenous phase for abdomen and pelvis) after the automatic injection of 120 mL of iodinated intravenous contrast agent (iopromide; Ultravist 370, Bayer Vital, Leverkusen, Germany, flow 2-3 mL/second) according to the routine clinical workflow. The CT series were performed in expiration, which enables better alignment to the PET series. Subsequently, the PET was performed, usually over 6-8 beds with 3 minutes per bed (axial FOV: 15.5 cm, slice thickness: 4.25 mm). During the whole PET/CT scan, the patients were positioned with raised arms in order to reduce beam-hardening artefacts.
The CT images were reconstructed using an axial slice thickness of 5 mm and coronal thickness of 3 mm, with increments of 5 mm and 2 mm, respectively. The PET scans were reconstructed with 4 iterations, 8 subsets, and a Gaussian filter of 4 mm. The PET and CT data were displayed simultaneously as separate and automatically fused images on a workstation provided by the vendor (TrueD, Siemens Health Care, Erlangen, Germany) in axial, coronal, and sagittal planes (PET matrix 128 × 128 pixels, CT matrix 512 × 512 pixels).
All PET/ceCT images were initially evaluated by a radiologist and a nuclear medicine specialist in consensus for the clinical report. The retrospective analysis of the incidental gastrointestinal findings started two months after the last included PET/CT examination, and the analysts were blinded to the initial results. The datasets of PET/ldCT and PET/ceCT were evaluated separately by two readers in consensus who were blinded to the results of endoscopy and histology.
All areas with suspicious elevated gastrointestinal FDG uptake were visually analysed regarding their localization in the upper or lower gastrointestinal tract (separated by the superior duodenal fold) and their FDG uptake pattern with differentiation into focal, segmental and diffuse uptake. FDG uptake with a spot-like, well-circumscribed appearance was defined as focal, whereas increased uptake usually with a longish shape comprising a large part of the circumference of the bowel in one segment was defined as segmental. Diffuse uptake was assigned if there was no circumscribed shape and inhomogeneous uptake, usually over several segments of the bowels. The SUVmax of the lesions was calculated using semi-automatic 3D region-of-interest analysis with 50% isocontour threshold of the maximum tracer uptake.
The morphological correlates of FDG uptake in ldCT and ceCT were classified into mass, inflammation, unspecific lesions, and areas without any abnormalities in CT based on their CT characteristics. A CT finding was categorized as mass if an intraluminal polypoid mass, a sharply circumscribed sessile mass, or circumferential thickening of the wall and contrast enhancement in ceCT were found. The category of inflammation was based on the CT characteristics diffuse thickening of the whole circumference of the bowel wall with contrast enhancement if applied, and reaction of the surrounding tissue. If these criteria were not met despite the existence of CT abnormalities, e.g. wall thickening that could not be clearly categorized as a mass or inflammation, the finding was categorized as an unspecific lesion.
The reference standard of the 60 patients was given by endoscopy in 58 patients, and by surgery in 2 patients. An additional histological examination was performed in 47 patients. A biopsy was not taken in 8 patients due to normal endoscopic results, and the remaining 5 patients without histology suffered from inflammatory bowel diseases and were controlled by clinical follow-up. Endoscopy was performed at an average of one month after PET/CT.
The endoscopic findings were classified into malignancies, premalignant masses, benign masses, inflammatory changes, and normal endoscopic results. All abnormal endoscopic findings, including the benign masses and the inflammatory diseases, were counted as pathological because of the need for further diagnostic investigation. Slight inflammatory changes without any clinical relevance were assigned to the group of normal endoscopic results. PET positive lesions with a suspicious finding in CT and a corresponding endoscopic abnormality were regarded as true positive. PET positive lesions with a correlative finding in CT but without endoscopic pathology were regarded as false positives. For the evaluation of the CT diagnosis, PET positive lesions without characteristic alterations in CT and without endoscopic abnormality were classified as true negatives, whereas PET positive lesions without a finding in CT but endoscopic pathology were classified as false negatives.
One-way ANOVA and Tukey test were used to assess whether there was a significant difference in the mean of SUVmax in the different subgroups (malignancy, premalignant mass, benign mass, inflammation, physiological accumulation). A p-value of less than 0.05 was considered to indicate statistical significance. The diagnostic performance of PET/ldCT and PET/ceCT was calculated using the positive predictive value (PPV), and if possible, with the additional use of the sensitivity. The significance of differences in the sensitivity was tested by the McNemar test. The statistical analysis was performed using JMP 8.0.2 software (SAS Institute, Cary, NC, USA).
Suspicious incidental gastrointestinal FDG uptake was found in 282 of 5045 patients with FDG-PET/CT scans, which corresponds to a rate of 5.6%. In the 60 patients finally included, 62 FDG avid lesions were found. 45 (72.6%) of these lesions corresponded to a pathological finding in endoscopy or surgery, whereas 17 lesions had no correlation in the reference examinations, and were thus interpreted as physiological FDG accumulation (Fig. 1). 17 of the 62 FDG avid lesions were located in the upper gastrointestinal tract, and 45 were found in the lower gastrointestinal tract.
The endoscopic and histological findings consisted of 17 (27.4%) malignancies, with primary cancers in the esophagus (n = 5), in the stomach (n = 1), in the ascending colon (n = 2), in the descending colon (n = 1), and in the sigmoid (n = 1), as well as in situ carcinoma in the sigmoid (n = 1), and metastases in the stomach (n = 2), in the small bowels (n = 1), in the transverse colon (n = 1), in the descending colon (n = 1), and in the sigmoid (n = 1). Furthermore, there were 10 (16.1%) premalignant lesions consisting of adenomas with intraepithelial low-grade neoplasia in the ascending colon (n = 4), in the transverse colon (n = 1), in the descending colon (n = 2), and in the sigmoid (n = 3), 5 benign masses with hyperplastic polyps in the ascending colon (n = 2) and the sigmoid (n = 2), and a vascular/lymphatic malformation in the transverse colon as well as 13 inflammations. The inflammatory changes in the upper gastrointestinal tract comprised candida esophagitis (n = 1), reflux esophagitis (n = 3), and erosive gastritis (n = 3), whereas changes in the lower gastrointestinal tract comprised drug-induced colitis in the sigmoid (n = 1), graft versus host disease in the sigmoid (n = 1), colitis ulcerosa (n = 1), abscess-forming diverticulitis in the descending colon (n = 1), infectious enteritis (n = 1), and an abscess formation of the rectum (n = 1). Premalignant and benign masses occurred only in the lower gastrointestinal tract, while malignant lesions and inflammations were found in both the upper and lower gastrointestinal tract (Fig. 2).
The relationship between the different FDG uptake patterns, the SUVmax of the lesions, and the different endoscopic subgroups is shown in Table 1. The 37 lesions with focal uptake in PET corresponded to an endoscopic mass in 23 cases (62.1%), an inflammation in 5 cases (13.5%), and a normal result in 9 cases (24.3%). Segmental uptake could be found throughout all subgroups in similar numbers. Diffuse uptake was caused by inflammation in 4 of 9 cases (44.4%), but represented false positive findings at the same percentage. One case with a diffuse uptake in the transverse colon concealed a premalignant adenoma within this region.
The mean SUVmax of the lesions with normal, inflammatory, benign, and premalignant endoscopic findings (7.0; 6.2; 7.3; 7.3) did not differ significantly. The range of the SUVmax in the normal, inflammatory, and benign lesions varied from 2.4 to a maximum of 10.2, in the group of premalignant lesions even up to a maximum of 22.1. The malignant lesions had a mean SUVmax of 13.7 (range: 5.6-29.4), which was significantly higher than the mean SUVmax of the normal, inflammatory, and premalignant subgroups. The difference to the few benign lesions was not significant (p = 0.0611).
The findings of ldCT and ceCT in the PET positive lesions in correlation to the endoscopic results are summarized in Table 2. The size of the masses found in CT ranged from 0.8-7.3 cm, and the morphologically visible wall thickenings in inflammations ranged from 0.6-1.7 cm. CeCT had a higher PPV for any pathologic findings than ldCT in the whole gastrointestinal tract, as well as in the separate evaluation of the upper and lower gastrointestinal tract (Table 3). CeCT detected all 17 malignancies, while ldCT identified only 5 of 17 malignancies correctly, and did not show any abnormal findings in 7 malignancies (41.2%). The sensitivity for the detection of a malignant lesion was 100% for ceCT and 29.4% for ldCT (p = 0.0001). The PPV of an abnormal mass in CT resulting in a malignant or premalignant finding in endoscopy was 81.5% for ceCT and 75.0% for ldCT. Premalignant lesions were detected in 50% by ceCT and in 10% by ldCT. Figure 3 shows one example of a carcinoma in situ with a sigmoid mass in ceCT but not in ldCT. The few benign endoscopic findings (n = 5) could not be identified correctly by either CT protocol.
Regarding inflammation, ceCT established the correct diagnosis in 6 of 13 cases (46.2%), with a relatively high number of false positive results (n = 5). One case of esophagitis with contrast enhancement and wall thickening in ceCT was falsely interpreted as a mass. None of the inflammatory lesions was identified correctly by ldCT. In regard to the 17 lesions with FDG accumulation in PET but normal endoscopic results, ceCT indicated true negatives in 10 lesions, whereas ldCT showed true negatives in 14 lesions.
The false negative rate for any pathology was 31.1% for ceCT and 68.9% for ldCT. The only false positive PET finding in the upper gastrointestinal tract with FDG uptake in the stomach also showed wall thickening in both CT protocols, and thus, could not be excluded by CT. In the lower gastrointestinal tract, ldCT had a lower false positive rate, which was observed in 2 of 16 lesions (12.5%), than ceCT, occurring in 6 of 16 lesions (37.5%).
The overall prevalence of suspicious incidental gastrointestinal FDG uptake in the PET/CT scans of the study group was 6%, which is higher than the rate of 1-3% reported by Israel et al. (1) and Kamel et al. (2). This is possibly due to the different inclusion criteria of the present study. In contrast to the previous studies, lesions were evaluated in the whole gastrointestinal tract with not only focal, but also segmental and diffuse FDG uptake patterns, irrespective of the CT findings. As with previous studies, a very high rate of premalignant or malignant endoscopic lesions were found (44%), especially in the upper gastrointestinal tract (1, 2, 8). 27% of the positive PET findings in the present study were false positives, which is in the range of the data in other studies, which had false positive rates of 13-33%, as far as the results can compared due to inconsistent inclusion criteria (1-3, 13).
Interestingly, more premalignant and malignant lesions were found than benign lesions in PET. This supports the hypothesis that the grade of dysplasia is an important predictive factor for elevated FDG uptake in the gastrointestinal tract (3, 14).
One approach to further characterise suspicious incidental gastrointestinal FDG uptake is the analysis of the FDG uptake pattern. In the present study, focal FDG uptake was suggestive for premalignant, malignant, and benign masses, which is in accordance with the literature (1, 2, 11). However, the present results indicate that focal inflammations and false positive focal uptake must not be neglected. Only a few studies have evaluated the relevance of segmental and diffuse FDG uptake patterns, and have found a correlation with inflammations and physiological processes (2, 8). Thirty-eight percent of the pathologic lesions in the present study displayed a segmental or diffuse uptake pattern, with a remarkable number of malignant lesions with segmental uptake (n = 6), and inflammations with diffuse uptake (n = 4). This contradicts the rigid correlation between uptake patterns and disease subgroups, and demonstrates that not only focal FDG uptake should be carefully considered as suspicious for malignancies or other relevant gastrointestinal pathologies. The mean SUV of malignant lesions in the present study was almost twice as high as the mean SUV of the other subgroups. The difference was significant for all subgroups except for the benign lesions, probably due to their small number of cases. Several studies found a tendency of the SUV increasing with progressive dyplasia in gastrointestinal lesions (3, 15). However, the findings remain inconsistent, and benign hyperplastic polyps particularly display a wide range from low FDG uptake to very high values up to SUV 25 (3, 16).
A dependency of the amount of FDG uptake on the size of the lesions has also been discussed (15). Based on the present results, the SUV can give a hint on the grade of malignancy of the underlying disease, but the wide overlap of the SUV values of benign and malignant lesions makes a decision in the individual case difficult.
Therefore, as PET alone does not allow for sufficient characterisation and localisation of suspicious incidental gastrointestinal findings, a question about the improvement of the diagnosis by the CT component in PET/CT protocols arises. The benefit of ceCT protocols in routine FDG-PET/CT is still under discussion (17, 18). In regard to incidental gastrointestinal uptake, Kamel et al. (2) showed that the CT characteristics help in the differentiation of benign an malignant lesions in a study with 42 malignant or premalignant lesions and 18 benign lesions in 69 patients. Kei et al. (11) found a soft-tissue abnormality in CT in 12 of 21 patients with incidental focal gastrointestinal FDG uptake. These studies were performed without intravenous contrast, whereas the present study aimed at evaluating the possible benefit of ceCT in this special situation, in accordance with the experience of Prabhakar et al. (12). It was found that ceCT with intravenous and oral contrast improved the detection and classification of underlying pathologies compared to the ldCT protocol (PPV 0.81 versus 0.70). All malignant lesions in the present study could be identified correctly by ceCT (sensitivity: 100%) compared to a sensitivity of 29% in ldCT. Also, the characteristics of ceCT predict a malignancy more exactly than the pattern of the FDG uptake. However, this was not confirmed for premalignant lesions and very early stages of cancer (CIS), as they were detected in only 50% by ceCT and in 10% by ldCT. Twenty-seven percent of the PET findings were false positives. All false positives except for one were located in the lower gastrointestinal tract. Additional CT could decrease this rate, but a substantial number of false positive cases still remained with both CT protocols. The larger number of false positives in ceCT compared to ldCT could possibly be caused by an overestimation of the contrast enhancement in contracted (and thus thickened) bowel walls, with a subsequent false interpretation as an inflammation or mass.
Regarding false negative findings, Kei et al. (11) and Gutman et al. (3) reported rates of 33% and 38%, respectively, for ldCT. Their data were based only on focal FDG uptake, where a correlation of possible CT changes with a PET positive lesion may be easier than in other uptake patterns. In the present study the false negative rate of ldCT for any pathology was 69%, and could be remarkably decreased by contrast enhancement, but still remained at 31% with ceCT. The ceCT protocol used in this study can decrease the number of false positive and false negative PET results, but cannot exclude pathologies in PET positive lesions, and therefore, endoscopy of an incidental gastrointestinal lesion cannot be forgone.
There are some limitations of the present study. First of all, in the inclusion process, as the PET/CT reports were considered for the searching of elusive findings, there could be a preselection of patients with overestimation of CT results. PET positive but CT negative findings may possibly be rated more often as physiological accumulation than cases with abnormal CT findings, and may therefore not be mentioned in the reports. Also, only patients with a PET/CT protocol comprising ldCT and ceCT in one examination and in-house endoscopy were included, which may have introduced some selection bias. However, about half of the patients in our institution were examined routinely with this protocol, and the intention of this study was to compare the CT protocols in the same patients directly. Furthermore, limitations were set by the ethical recommendations concerning the obtainment of reference standard examinations. Also, in the time interval between PET/CT examination and endoscopy, the appearance of inflammations might have changed in individual cases. The number of lesions in the different subgroups was limited. The subgroup of malignancies was relatively small (n = 17), and PET positive malignancies with no CT abnormalities might be found in larger collectives.
In conclusion, the integration of ceCT in combined PET/CT protocols cannot replace the subsequent endoscopic examination of incidental gastrointestinal PET positive lesions, because of a substantial number of false negative results. Therefore, it cannot be recommended to perform ceCT in PET/CT routinely for further differentiation of possible gastrointestinal PET positive lesions, or to add ceCT to a PET/ldCT protocol after the detection of suspicious incidental gastrointestinal FDG uptake. If a CT contrast agent is applied for clinical reasons in PET/CT, the CT pattern can help to guide the further work-up of incidental gastrointestinal PET lesions, especially with suspicion of gastrointestinal malignancies.
Figures and Tables
Fig. 1
Flow diagram shows selection process and final diagnosis of 60 patients with suspicious incidental gastrointestinal FDG uptake. ldCT = low-dose CT, ceCT = contrast-enhanced CT, GIT = gastrointestinal tract

Fig. 2
Distribution lesion number in subgroups of endoscopic lesions differs in different parts of gastrointestinal tract.

Fig. 3
Seventy two-year-old male patient with bronchial carcinoma in left lower lobe presented with suspicious incidental gastrointestinal FDG uptake in sigmoid colon.
MIP of PET shows primary tumour in lung (black arrow) and suspicious incidental gastrointestinal FDG uptake in sigmoid (white arrow) (A). Axial PET (B) and axial PET/CT fusion (C) show focal FDG uptake in sigmoid. No correlate was found in non-enhanced low-dose CT (D), whereas contrast-enhanced CT showed corresponding intraluminal mass (1.3 cm) (white arrow) (E). Endoscopy revealed adenomatous mass with carcinoma in situ in histological examination (F).
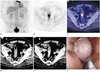
Table 1
Correlation of FDG Uptake Patterns and SUV with Reference Standard in 60 Patients with 62 Suspicious Incidental Gastrointestinal Lesions

References
1. Israel O, Yefremov N, Bar-Shalom R, Kagana O, Frenkel A, Keidar Z, et al. PET/CT detection of unexpected gastrointestinal foci of 18F-FDG uptake: incidence, localization patterns, and clinical significance. J Nucl Med. 2005; 46:758–762.
2. Kamel EM, Thumshirn M, Truninger K, Schiesser M, Fried M, Padberg B, et al. Significance of incidental 18F-FDG accumulations in the gastrointestinal tract in PET/CT: correlation with endoscopic and histopathologic results. J Nucl Med. 2004; 45:1804–1810.
3. Gutman F, Alberini JL, Wartski M, Vilain D, Le Stanc E, Sarandi F, et al. Incidental colonic focal lesions detected by FDG PET/CT. AJR Am J Roentgenol. 2005; 185:495–500.
4. Delbeke D. Oncological applications of FDG PET imaging: brain tumors, colorectal cancer, lymphoma and melanoma. J Nucl Med. 1999; 40:591–603.
5. Engel H, Steinert H, Buck A, Berthold T, Huch Böni RA, von Schulthess GK. Whole-body PET: physiological and artifactual fluorodeoxyglucose accumulations. J Nucl Med. 1996; 37:441–446.
6. Strauss LG. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med. 1996; 23:1409–1415.
7. Abouzied MM, Crawford ES, Nabi HA. 18F-FDG imaging: pitfalls and artifacts. J Nucl Med Technol. 2005; 33:145–155. quiz 162-163.
8. Tatlidil R, Jadvar H, Bading JR, Conti PS. Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathologic findings. Radiology. 2002; 224:783–787.
9. Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005; 46:752–757.
10. Drenth JP, Nagengast FM, Oyen WJ. Evaluation of (pre-) malignant colonic abnormalities: endoscopic validation of FDG-PET findings. Eur J Nucl Med. 2001; 28:1766–1769.
11. Kei PL, Vikram R, Yeung HW, Stroehlein JR, Macapinlac HA. Incidental finding of focal FDG uptake in the bowel during PET/CT: CT features and correlation with histopathologic results. AJR Am J Roentgenol. 2010; 194:W401–W406.
12. Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA. Bowel hot spots at PET-CT. Radiographics. 2007; 27:145–159.
13. Yasuda S, Fujii H, Nakahara T, Nishiumi N, Takahashi W, Ide M, et al. 18F-FDG PET detection of colonic adenomas. J Nucl Med. 2001; 42:989–992.
14. van Kouwen MC, Nagengast FM, Jansen JB, Oyen WJ, Drenth JP. 2-(18F)-fluoro-2-deoxy-D-glucose positron emission tomography detects clinical relevant adenomas of the colon: a prospective study. J Clin Oncol. 2005; 23:3713–3717.
15. Weston BR, Iyer RB, Qiao W, Lee JH, Bresalier RS, Ross WA. Ability of integrated positron emission and computed tomography to detect significant colonic pathology: the experience of a tertiary cancer center. Cancer. 2010; 116:1454–1461.
16. Abdel-Nabi H, Doerr RJ, Lamonica DM, Cronin VR, Galantowicz PJ, Carbone GM, et al. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology. 1998; 206:755–760.
17. Elstrom RL, Leonard JP, Coleman M, Brown RK. Combined PET and low-dose, noncontrast CT scanning obviates the need for additional diagnostic contrast-enhanced CT scans in patients undergoing staging or restaging for lymphoma. Ann Oncol. 2008; 19:1770–1773.
18. Pfannenberg AC, Aschoff P, Brechtel K, Müller M, Klein M, Bares R, et al. Value of contrast-enhanced multiphase CT in combined PET/CT protocols for oncological imaging. Br J Radiol. 2007; 80:437–445.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download