Abstract
We report MR imaging findings of a rare case of endocervical mucinous borderline tumor (MBT) involving the cul-de-sac and left fallopian tube arising from extensive pelvic endometriosis with pathologic correlation in a 35-year-old woman presented with vague pelvic pain. Endocervical MBT is a type of endometriosis-associated carcinoma. Imaging findings of endocervical MBT are unilocular or oligolocular cystic lesions with enhancing mural nodules, which are different from those of the more common intestinal type MBT.
Endometriosis is a common gynecologic condition which is characterized by the presence of endometrial tissue implants composed of endometriotic glands and stroma outside the uterus. Endometriosis is considered to be a precancerous disease, and approximately 2.5% of women with endometriosis develop an endometriosis-associated ovarian cancer (1). Endometrioid and clear cell carcinomas are the most commonly reported histologic types of endometriosis-associated ovarian cancers (2, 3), but endocervical MBTs are also reported to be endometriosis-associated tumors that are much less common than endometrioid or clear cell tumors. To the best of our knowledge, there is no literature on the imaging features of extraovarian endocervical MBT (4, 5). Therefore, we report a rare case of extraovarian endocervical MBT involving the cul-de-sac and fallopian tube associated with extensive pelvic endometriosis.
A 35-year-old woman (gravida 0, para 0, abortus 0) presented with dysmenorrhea and vague lower abdominal pain. Her past history was unremarkable. At the time of presentation, she was having regular menstrual cycles. She was afebrile and her abdomen was soft and flat without any tender points. Laboratory data were normal except for the elevated level of CA 125 (44 U/mL; normal 0-35 U/mL) and CA 19-9 (271 U/mL; normal 0-37 U/mL).
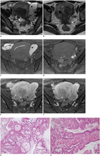
MRI showed an elongated tubular cystic lesion with high signal intensity on both T1- and T2-weighted images in the left adnexa, which was diagnosed as hematosalpinx. An axial T2-weighted image showed a stellate low signal intensity lesion with obliteration of the cul-de-sac, suggestive of deep infiltrating endometriosis. The uterus was diffusely enlarged with focal adenomyosis and an intraumral myoma in the posterior wall. Two small cystic lesions with mural nodules were present in the cul-de-sac along the posterior margin of the uterus. These cystic components showed high signal intensity similar to that of urine on T2-weighted images as well as high signal intensity that was slightly higher than that of myometrium on fat-suppressed T1-weighted images. Peripheral nodular enhancement was seen in the cystic lesions on gadolinium-enhanced T1-weighted images, raising the possibility of malignancy (Fig. 1). Mural nodules were not definitely seen in the left fallopian tube.
Surgery revealed severe pelvic endometriosis involving bilateral ovaries, the cul-de-sac, rectosigmoid colon and left hydrosalpinx. On histopathologic examination, cul-de-sac masses and the left fallopian tube showed mucin-filled cysts and branching papillary projections. The papillae were lined by endocervical type mucinous cells. Microscopic examination revealed a gradual transition from the epitheliums of endometeriosis to mucinous tumor cells. The pathologic diagnosis was confirmed as endocervical mucinous boderline tumor involving the cul-de-sac and left fallopian tube arising from extensive pelvic endometriosis.
Endometriosis, like cancer, is characterized by growth of blood vessels (angiogenesis) and a decrease in the number of cells undergoing apoptosis (6). Therefore, endometriosis is considered as a possible precancerous disease. Malignant transformations have been reported in women with endometriosis with a frequency of about 2.5%; with 80% in the ovary and 20% in the extraovarian sites (2, 7, 8). Extraovarian endometriosis-associated cancers were reported to occur in various anatomic locations in the following frequencies: rectovaginal sites (36%), colorectum (11%), bladder (9%), vagina (7%), pelvic ligaments (4%), umbilicus (4%), cervix (4%) and fallopian tube (4%) (7).
The histopathologic criteria for the diagnosis of endometriosis-associated ovarian carcinoma, first proposed by Sampson in 1924, require a combination of the following three findings: 1) demonstration of both cancerous and benign endometrial tissues in the tumor, 2) histology of the neoplasm is compatible with an endometrial origin, and 3) no other primary tumor sites affected. These criteria are still used and extended to extraovarian sites (8). In addition, Scott added the strict criteria of the presence of the dysplastic phase or transition zone between the benign endometriosis and the carcinoma, but the transition zone is only detected in 36-42% of cases at the present time (8, 9). Although much less common than clear cell or endometrioid carcinomas, endocervical MBT is known to be associated with pelvic endometriosis (10).
Ovarian mucinous tumors are currently classified into two subtypes based on pathologic findings: intestinal type and endocervical or müllerian type (11). Intestinal type mucinous ovarian tumors are much more common than endocervical type tumors, and nearly all reports of mucinous ovarian tumors describe the former. However, endocervical MBTs show different clinicopathologic features from intestinal MBTs. First, intestinal MBTs are bilateral in only 0-10%, whereas endocervical MBTs are commonly bilateral (12-47%). Secondly, intestinal types are almost never associated with endometriosis, but endocervical types are often associated with endometriosis (30-53% of cases). Thirdly, intestinal types are usually multilocular cysts without papillary features, whereas endocervical types are unilocular or oligolocular cysts with fine papillary projections, resembling serous borderline tumors or malignant tumors arising in endometriosis such as endometrioid or clear cell carcinomas.
The imaging findings of intestinal and endocervical MBTs are also quite different. The well-known imaging features of ovarian MBTs are multilocular cystic lesions with variable signal intensities in each locule, known as stained glass appearance, which may reflect the imaging findings of more common intestinal MBTs. In contrary, imaging findings of endocervical MBTs in our case and from other reports show unilocular or paucilocular cystic lesions with papillary projections or mural nodules corresponding to the gross features (4, 12). However, these findings can also be seen in clear cell or endometrioid borderline tumors as well as in carcinomas (5). Kataoka et al. (4) suggested mural nodules in three cases of endocervical MBTs showing marked high signal intensity on T2-weighted MR images due to prominent mucin and stromal edema, which may be helpful to differentiate endocervical mucinous borderline tumors from other carcinomas arising from endometriotic cysts. However, Tanaka et al. (13) analyzed 49 cases of ovarian endometriotic cysts and revealed that both benign and malignant mural nodules may show high signal intensity on T2-weighted images. Also, the mural nodules in our case did not show striking high signal intensity on T2-weighted images. Therefore, it may be difficult to differentiate borderline ovarian tumors from invasive carcinomas based on the signal intensity of mural nodules. Our case is different from previous reports in that the endocervical MBT occurred in the extraovarian site including the cul-de-sac and fallopian tube, but the imaging findings of extraovarian endocervical MBTs were similar to those of ovarian endocervical MBTs.
The prognosis of endocervical MBTs is much better than clear cell or endometrioid carcinomas arising from endometriotic cysts (4). However, endocervical MBT is of clinical significance because the tumor occurs more often in young, nulliparous or infertile women, many of whom wish to preserve their fertility and thus agree to only limited surgery such as cystectomy (10). If preservation of potential fertility is supreme, then careful inspection of both the remaining and contralateral ovary for any residual tumor or endometriotic foci is critical, as these may be future sources of recurrent tumors.
In summary, endocervical MBT is an additional tumor type arising from endometriosis and can involve the ovary as well as extraovarian sites. Imaging findings of endocervical MBT arising from endometriosis involving the ovary or extraovarian sites are unilocular or oligolocular cystic lesions with enhancing mural nodules, which are quite different from those of the more common intestinal type MBT. Although the imaging findings of endocervical MBT are not specific, radiologists should keep in mind that extraovarian involvement of MBT can occur in patients with endometriosis.
Figures and Tables
Fig. 1
Thirty five-year-old woman with extraovarian endocervical mucinous borderline tumor arising from pelvic endometriosis.
Consecutive axial T2-weighted (A, B), fat-suppressed T1-weighted (C, D) and contrast-enhanced fat-suppressed T1-weighted (E, F) MR images. MR images show elongated tubular cystic lesion (asterisk in A, C) with high signal intensity on both T1- and T2-weighted images in left adnexa, which is diagnosed as hematosalpinx. Axial T2-weighted image (B) shows stellate low signal intensity lesion (arrow in B) with obliteration of cul-de-sac, suggestive of deep infiltrating endometriosis. Uterus is diffusely enlarged with focal adenomyosis and intramural myoma in posterior wall. Two small cystic lesions (arrowheads in A, D) with high signal intensity on T2-weighted image and slightly high signal intensity on T1-weighted image are present in cul-de-sac along posterior margin of uterus. Following contrast-infusion, peripheral nodular enhancement is noted in cystic lesions (arrowheads in E, F), raising possibility of malignancy. Histopathologic findings of cul-de-sac masses show multiple mucin-filled cysts (asterisks in G) and papillary projections (arrows in G) lined by endocervical type mucinous cells. These masses were confirmed as endocervical mucinous borderline tumors. Gradual transition from epitheliums of endometriosis (white arrow in H) to mucinous tumor cells (black arrow in H) was identified.
References
1. Melin A, Sparén P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod. 2006; 21:1237–1242.
2. Stern RC, Dash R, Bentley RC, Snyder MJ, Haney AF, Robboy SJ. Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol. 2001; 20:133–139.
3. Yoshikawa H, Jimbo H, Okada S, Matsumoto K, Onda T, Yasugi T, et al. Prevalence of endometriosis in ovarian cancer. Gynecol Obstet Invest. 2000; 50:Suppl 1. 11–17.
4. Kataoka M, Togashi K, Koyama T, Yamaoka T, Iwasa Y, Fujii S, et al. MR imaging of müllerian mucinous borderline tumors arising from endometriotic cysts. J Comput Assist Tomogr. 2002; 26:532–537.
5. McDermott S, Oei TN, Iyer VR, Lee SI. MR imaging of malignancies arising in endometriomas and extraovarian endometriosis. Radiographics. 2012; 32:845–863.
6. Bedaiwy MA, Hussein MR, Biscotti C, Falcone T. Pelvic endometriosis is rarely associated with ovarian borderline tumours, cytologic and architectural atypia: a clinicopathologic study. Pathol Oncol Res. 2009; 15:81–88.
7. Brooks JJ, Wheeler JE. Malignancy arising in extragonadal endometriosis: a case report and summary of the world literature. Cancer. 1977; 40:3065–3073.
8. Benoit L, Arnould L, Cheynel N, Diane B, Causeret S, Machado A, et al. Malignant extraovarian endometriosis: a review. Eur J Surg Oncol. 2006; 32:6–11.
9. Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953; 2:283–289.
10. Kim KR, Choi J, Hwang JE, Baik YA, Shim JY, Kim YM, et al. Endocervical-like (Müllerian) mucinous borderline tumours of the ovary are frequently associated with the KRAS mutation. Histopathology. 2010; 57:587–596.
11. Shappell HW, Riopel MA, Smith Sehdev AE, Ronnett BM, Kurman RJ. Diagnostic criteria and behavior of ovarian seromucinous (endocervical-type mucinous and mixed cell-type) tumors: atypical proliferative (borderline) tumors, intraepithelial, microinvasive, and invasive carcinomas. Am J Surg Pathol. 2002; 26:1529–1541.
12. Rutgers JL, Scully RE. Ovarian mullerian mucinous papillary cystadenomas of borderline malignancy. A clinicopathologic analysis. Cancer. 1988; 61:340–348.
13. Tanaka YO, Okada S, Yagi T, Satoh T, Oki A, Tsunoda H, et al. MRI of endometriotic cysts in association with ovarian carcinoma. AJR Am J Roentgenol. 2010; 194:355–361.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download