Abstract
Objective
Materials and Methods
Results
Figures and Tables
 | Fig. 1Mean CT number of four different tissues in 44 patients with different SAFIRE strengths and previous CT scans (group B). Hounsfield units for different SAFIRE strengths (0 to 5) show no significant difference for aorta (p = 0.999), liver (p = 0.995), muscle (p = 0.984) and fat (p = 0.929). Compared to prior CTs, CT numbers were significantly increased due to application of CARE kV (i.e., 100 kVp instead of 120 kVp) for aorta (23.8%, p < 0.001), liver (18.6%, p < 0.001) and muscle (10.0%, p < 0.001) and were increased for fat (10.9%, p < 0.001). SAFIRE = sinogram-affirmed iterative reconstruction |
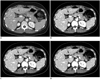 | Fig. 2Forty-three-year-old male with small hepatic cysts.
A. Image scanned with 120 kVp and reference mAs of 240 and reconstructed with FBP, resulting in effective dose 8.69 mSv. B, C and D. CT scan obtained 2 months later with CARE kV (i.e., 100 kVp) and 30% reduced reference mAs of 170, resulting in effective dose of 4.74 mSv (45.5% dose reduction) and reconstructed without application of SAFIRE (i.e., use of FBP); (B) and with SAFIRE strengths of 3 (C) and 5 (D). Image noise was 7 HU (A), 12 HU (B), 8.7 (C) and 4.8 (D), respectively. FBP = filtered back projection, SAFIRE = sinogram-affirmed iterative reconstruction, HU = Hounsfield Unit
|
Table 1

Note.- Group A (reference mAs 240, 120 kVp, and filtered back projection vs. reference mAs 240, CARE kV [i.e., 100 kVp] and filtered back projection). Group B (reference mAs 240, 120 kVp, and filtered back projection vs. reference mAs 170, CARE kV [i.e., 100 kVp] and SAFIRE). Body weight was not significantly different between time of study the time of prior CT in both group A (p = 0.278) and group B (p = 0.287). CT = computed tomography, SAFIRE = sinogram-affirmed iterative reconstruction
Table 2

Note.- Reference mAs of 240, 120 kVp, and filtered back projection for prior CT vs. reference mAs of 240, CARE kV (i.e., 100 kVp) and filtered back projection for CT with CARE kV. Organ attenuation and organ noise were obtained by taking average and SD, respectively, of ROI measurement of CT attenuation. *Numbers in parentheses indicate average change between prior CT and CT during current study time. CT = computed tomography, HU = Hounsfield Unit, SD = standard deviation, ROI = region of interest
Table 3

Note.- Organ noise was obtained by taking SD of ROI measurement of CT attenuation in each tissue/organ. *P values result from comparing images reconstructed with each SAFIRE strength and prior CT images reconstructed with FBP. Numbers in parentheses indicate average image noise change between prior CT and CT reconstructed with each SAFIRE strength. CT = computed tomography, SAFIRE = sinogram-affirmed iterative reconstruction, HU = Hounsfield Unit, FBP = filtered back projection, ROI = region of interest, SD = standard deviation
Table 4
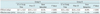
Note.- Group A (reference mAs 240 and 120 kVp without CARE kV vs. reference mAs 240 and 100 kVp with CARE KV), Group B (reference mAs 240 and 120 kVp without CARE kV, SAFIRE vs. reference mAs 170 and 100 kVp with CARE kV and SAFIRE). CT = computed tomography, SAFIRE = sinogram-affirmed iterative reconstruction




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download