Abstract
Optical coherence tomography (OCT) is a new imaging technique capable of obtaining high-resolution intravascular images and has been used in interventional cardiology. However, an application of OCT in pulmonary arteries had seldom been documented. In this case, OCT imaging is performed in peripheral pulmonary arteries and shows mural red thrombi. Subsequently, the red thrombi are aspirated and confirmed by a histological examination. These findings suggest that OCT may be a useful tool to depict peripheral pulmonary artery thrombi.
With the development of the multi-detection computed tomography (MDCT), the MDCT pulmonary angiography (MDCTA) has become the first-line technology for diagnosing pulmonary embolism (PE) (1); however, this technique does not allow for a direct visualization of intravascular lesions and vessel walls. Optical coherence tomography (OCT) is a relatively new imaging technique which has been used to characterize a variety of intravascular disorders, particularly for coronary artery (2). Here, we report the use of the OCT technique in order to visualize red thrombi located in peripheral pulmonary arteries of patients with PE.
A 42-year-old man was transferred to our institution for emergency treatment. He had experienced acute occlusion of the left and right pulmonary arteries caused by a rupture of a massive right atrial thrombus as confirmed by MDCTA (Fig. 1A, C). He received thrombolytic therapy with intravenous administration of 100 mg recombinant tissue plasminogen activator, followed by anticoagulation with subcutaneous lower-molecular-weight heparin. Anticoagulation treatment with oral warfarin was consistently administered. After being discharged one month later, he had shortness of breath under moderate-intensity exertion and was readmitted. MDCTA was performed and the result revealed a complete resolution of the thrombi in both main pulmonary arteries as well as an inconclusive filling defect sign in the peripheral pulmonary artery of the right lower lobe (Fig. 1B, D). To explore pulmonary arteries, pulmonary angiography and selective pulmonary angiography were performed via the right femoral approach, using a 6 Fr sheath, pig tail and multipurpose catheters, respectively. The results revealed several filling defects in the subsegmental pulmonary arteries of both the lower and right upper lobes (Fig. 1F, G). Then, OCT was performed to evaluate these intravascular lesions. An OCT system (Model M2 Cardiology Imaging System, LightLab Imaging, Inc., Westford, MA, USA) with a motorized pull-back system was used. A 0.016-inch OCT image wire (ImageWire, LightLab Imaging, Inc.) was advanced to the distal end of the lesions through a 3 Fr occlusion balloon catheter. In order to remove the blood as well as to obtain clear images, the occlusion balloon was inflated with an inflated device, with the inflating pressure ranging from 0.4 to 1.0 atm (1 atm = 101.3 kPa) at the proximal site of the vessel. Lactate Ringer's solution was infused into the artery from the distal tip of the occlusion balloon catheter at 1.0 to 2.0 mL·s-1. Motorized pullback OCT imaging was performed at a rate of 1.0 mm·s-1 for a length of 30 mm. Images were acquired at 15 frames·s-1 and were digitally archived. The OCT images revealed mostly total occlusive mural protrusions (Fig. 1I). After OCT imaging, a multipurpose catheter was advanced to the opening of the pulmonary artery and the proximal connected syringe and was slowly retracted. These retrieved materials were dark red strip-like tissue (Fig. 1K), which were fixed in formalin and processed for staining with hematoxylin and eosin. The result of the histological examination was red thrombi (Fig. 1L). An immediate check-up angiography showed the disappearance of the filling defects (Fig. 1H). OCT was performed again and demonstrated no mural protrusion (Fig. 1J). After a half-year of anticoagulation treatment with warfarin, his symptoms were resolved.
Currently, there are two types of intravascular imaging techniques to visualize the intravascular emboli and vessel wall in vivo. The intravascular ultrasound (IVUS), introduced in the late 1980s, has been demonstrated to acquire detailed cross-sectional images of coronary arteries (3). It has been reported that IVUS clarified the pulmonary emboli located in the main pulmonary arteries in patient with pulmonary emboli (4). OCT is a recently developed light-based imaging technique analogue to IVUS. Due to its shorter wavelength of infrared light, OCT provides a higher image resolution (10 to 20 µm) and penetration (2 to 3 mm), which is referred to as 'optical biopsy'. Currently, many studies had demonstrated that IVUS is not an efficient instrument to visualize the intravascular small lesions and the microstructure of vessels as compared to the OCT due to its relatively lower image resolution (3, 5). OCT signals are attenuated by red blood cells; the red thrombus contains more red blood cells than white one. Therefore, the OCT allows not only a morphology assessment of the thrombi, but also the ability to differentiate red from white thrombi in the coronary arteries (6) and peripheral pulmonary arteries (7). Moreover, the sufficient length of imagewire with the OCT M2 system (191 cm) and the fine outer diameter of the tip imagewire (only 0.016-inch) may suggest that OCT is more suitable for imaging in small and distal vessels than other in vivo imaging technology currently available (8).
In fact, there have been a few studies of peripheral pulmonary arteries with OCT in patients. The clinical significance of utilizing OCT in pulmonary circulation is still uncertain. In our opinion, OCT can be used to diagnose pulmonary vascular disorders, because the technique provides higher resolution intraluminal cross-sectional and consecutive images, which allows for the differentiation of intraluminal lesion from extraluminal compression. In addition, thickened intima of peripheral pulmonary artery in patients with idiopathic pulmonary hypertension (IPH) was visualized with OCT, which may assist in the diagnosis of IPH (9). Pulmonary thrombi usually come from deciduous leg clots. Sevitt (10) studied 50 thrombi in the femoral vein and found that most thrombi structure has two main regions, red and white areas. The red region is usually dominated by red cells and fibrin, whereas the white region is characterized by many foci of platelets with fibrin border. The red thrombi are usually considered as fresh thrombi and are easily resolved by thrombolytic. Kume et al. (6) analyzed coronary arterial thrombi with OCT and found that the OCT signal attenuation of the red thrombi is more significant than the white ones, which can be used to differentiate red thrombus from the white one with sensitivity of 90% and specificity of 88%. The present case conveys OCT images of red thrombi in the peripheral pulmonary artery, which are finally confirmed by a histologic examination and are similar to OCT manifestation of red thrombi in Kume's study. Therefore, OCT could be used to differentiate red thrombus from white one as well as in deciding the next treatment plan in clinical practice.
The OCT technique may be a promising tool for diagnosing pulmonary vascular disorders. With additional studies of OCT in pulmonary vascular diseases, more clinical indications will be discovered.
Figures and Tables
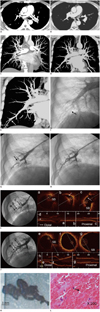 | Fig. 1Computed tomography pulmonary angigraphy images before and after thrombolytic and anticoagulation therapy (A-D), and detection of peripheral arterial thrombi at the right lower lung (E-H), and OCT images and pathological study of the thrombus (I-L).
Cross-section and coronal multiplanar reconstruction multi-detection computed tomography pulmonary angiography images of chest. (A) and (C) demonstrate multiple filling defects (black arrows) in left and right pulmonary arteries and enlargement of main pulmonary before treatment. (B) shows no clots in central pulmonary arteries, but shows inconclusive filling defect in peripheral pulmonary artery of right lower lobe, which is shown in (D) (white arrow), after thrombolysis and subsequent anticoagulation therapy. Correlation between coronal multi-detection computed tomography pulmonary angiography (MDCTA) image, pulmonary artery (PA) angiogram and selective pulmonary artery (SPA) angiogram. (E) shows post-treatment check MDCTA image, inconclusive filling defect in peripheral PA of right lower lobe is demonstrated with white arrow and matches with filling defect sign with black arrow is shown in (F) in PA angiogram and (G) in SPA angiogram, respectively. (H) shows same peripheral PA, which is recanalized after thrombus aspiration. Correlation between selective pulmonary segmental artery angiograms and optical coherence tomography (OCT) cross-sectional and longitudinal images at baseline and after thrombus aspiration. Basal angiogram (I) shows nearly total occlusion of lateral-basal segment of right lower lobe in contrast to corresponding OCT cross-sectional images (a, b, and c) and longitudinal view (d). OCT cross-sectional image (a) shows side branch (SB) that is used as landmark to locate corresponding sites between angiogram and OCT images. Images (b and c) show massive protruding into lumen (arrow). Image (d) shows lumen is obstructive and sites corresponding to images (a, b, and c) are identified by white lines, respectively. After thrombus aspiration, image (J) shows that no filling defect is observed and artery lumen is unobstructed; these corresponding sites OCT images are shown in right lower panel. OCT cross-sectional image (e, f, and g) and longitudinal view (h) corresponds to images (a, b, c, and d), respectively. OCT cross-sectional image (e) shows SB and images (f, g, and h) clearly demonstrate disappearance of protrusions and enlarged lumen, compared with images (b, c, and d), respectively. Corresponding sites to images (e, f, and g) are marked in image (h) with white lines, respectively. Gross appearance and histologic examination of retrieved materials from catheter. (K) shows that these retrieved materials appear as dark-red strip-like structure (scale bar 3 mm). Histologic examination in (L) shows that these materials primarily consist of red blood cells (arrow).
|
References
1. Schoepf UJ, Costello P. CT angiography for diagnosis of pulmonary embolism: state of the art. Radiology. 2004; 230:329–337.
2. Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010; 31:401–415.
3. Rieber J, Meissner O, Babaryka G, Reim S, Oswald M, Koenig A, et al. Diagnostic accuracy of optical coherence tomography and intravascular ultrasound for the detection and characterization of atherosclerotic plaque composition in ex-vivo coronary specimens: a comparison with histology. Coron Artery Dis. 2006; 17:425–430.
4. Tapson VF, Davidson CJ, Kisslo KB, Stack RS. Rapid visualization of massive pulmonary emboli utilizing intravascular ultrasound. Chest. 1994; 105:888–890.
5. Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007; 50:933–939.
6. Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006; 97:1713–1717.
7. Hong C, Wang W, Zhong NS, Zeng GQ, Wu H. Using optical coherence tomography to detect peripheral pulmonary thrombi. Chin Med J (Engl). 2012; 125:3171–3174.
8. Regar E, Schaar JA, Mont E, Virmani R, Serruys PW. Optical coherence tomography. Cardiovasc Radiat Med. 2003; 4:198–204.
9. Hou J, Qi H, Zhang M, Meng L, Han Z, Yu B, et al. Pulmonary vascular changes in pulmonary hypertension: optical coherence tomography findings. Circ Cardiovasc Imaging. 2010; 3:344–345.
10. Sevitt S. The structure and growth of valve-pocket thrombi in femoral veins. J Clin Pathol. 1974; 27:517–528.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download