Abstract
Pheochromocytoma is a rare cause of hypertension in children. Hypertension is one of the common reasons of posterior reversible encephalopathy. Intracerebral hemorrhage is a serious and unexpected complication of hypertensive encephalopathy due to pheochromocytoma, and very rarely seen in the childhood. Intracerebral hemorrhages should be searched if there are hypertensive reversible signal changes on the brain. Susceptibility weighted imaging (SWI) is a more sensitive method than conventional MRI when demonstrating cerebral microhemorrhagic foci. This is the first report of SWI findings on intracerebral hemorrhages in basal ganglia, brain stem and periventricular white matter due to hypertensive encephalopathy in a child with pheochromocytoma.
The most common causes of spontaneous intracranial hemorrhage in children are vascular malformations. Other relatively frequent reasons are hypertension, hematological disorders, and cerebral tumors. In general, hypertension in childhood is due to renovascular abnormalities, renal parenchymal diseases, and endocrinal disorders. Pheochromocytoma is one of the endocrinal diseases causing hypertension. Patients are usually presented with catecholamine excess signs and symptoms (1). Pheochromocytoma is a very rare cause of intracerebral hematoma in children, and few cases regarding brain involvement of pheochromocytoma have been reported so far.
Susceptibility weighted imaging (SWI) is a more sensitive method than conventional MRI for demonstrating cerebral microhemorrhagic foci. SWI is widely used in traumatic brain injury, amyloid angiopathy, and recently, posterior reversible encephalopathy syndrome (PRES) (2).
Herein, we present a child with hypertensive encephalopathy and intracerebral hematoma due to adrenal pheochromocytoma. On the SWI, multiple foci of hemorrhages that were not seen from the previous conventional MRIs were detected. This is the first report of SWI findings of intracerebral hemorrhages in basal ganglia, brain stem and periventricular white matter due to hypertensive encephalopathy for a child with pheochromocytoma.
A 13-year-old girl was presented with flushing and swelling on her hands and feet, sweating and critical headache for one year. On her medical history; she had previously been admitted for similar complaints and underwent surgery. Right adrenalectomy was made seven years ago and pathology result was pheochromocytoma. She had been discharged with a cure and a recommendation of interval follow-ups. But, the patient did not attend any follow-up visits nor receive any treatments over the last seven years. She started to complain of similar symptoms again since last year. On physical examination; she had cyanosis and edema on both hands and feet. The patient's blood pressure was 215/165 mm Hg, and her pulse rate was 137 beats per minute. Biochemical and radiological examinations were performed to determine recurrent pheochromocytoma. Urinary and plasma catecholamine levels were high. Urinary normetanephrine level was 30084 µg/24 h (reference values are 63-402 µg/24 h), urinary metanephrine level was 1452 µg/24 h (reference values are 32-167 µg/24 h), plasma noradrenalin level was 17540 pg/mL (reference values are 0-400 pg/mL). Contrast enhanced abdominal MRI was performed with a large mass within the left adrenal gland, measuring 65 × 50 mm in size. It was hypointense on T1-weighted and heterogeneous hyperintense on T2-weighted image with intense enhancement on post-contrast images (Fig. 1A), suggesting residue or recurrence of the adrenal pheochromocytoma. Cranial MRI was performed because of serious headaches. T2-weighted and fluid attenuated inversion recovery (FLAIR) images showed abnormal hyperintense signals on bilateral caudate, lentiform nuclei, dentate nuclei and periventricular and deep white matter (Fig. 1B-D). Furthermore, there were two hematomas; a chronic hematoma with peripheral hemosiderin rim on the right external capsule measuring 3 × 1 cm in size and a subacute hematoma on the right globus pallidus measuring 1 cm in diameter (Fig. 1C, E). There was increased diffusion in the lesions consistent with the vasogenic edema. These findings were consistent with severe PRES for the involvement of periventricular and deep white matter and deep gray matter as well as parenchymal hematomas.
On the tenth day of anti-hypertensive therapy, we obtained follow-up cranial MRI with additional SWI sequence. There was remarkable regression of vasogenic edema with near completed resolution of T2 high signal intensities on periventricular and deep white matter, and complete loss of edema on caudate, lentiform, and dentate nuclei (Fig. 1F-H). On SWI, many millimetric foci of hypointensity suggesting micro hemorrhages were observed on bilateral basal ganglia, periventricular white matter and brain stem (Fig. 1I). These microhemorrhagic foci were not detectable on conventional MRI. Reversible high signal intensities supported that this condition was PRES secondary to hypertension. SWI sequence revealed many micro and macro hemorrhagic foci of hypertensive encephalopathy as a complication of untreated chronic hypertension.
Pheochromocytomas are neuro-endocrine tumors arising from chromoffin cells of the adrenal medulla or the extraadrenal paraganglia. Producing and excreting a large amount of cathecolamine is typical for these tumors. The classical symtoms are due to blood cathecolamine excess; headache, hypertension, hyperhydrosis, hyperglycemia, and hypermetobolism. Complications of the pheochromocytoma are rarely seen, such as cardiac arrhythmias, myocardial infarction and sudden deaths areassociated with cardiotoxic effects of high cathecolamine levels (3). Intracerebral ischemia, infarction and hemorrhage are other serious complications, related with increased platelet aggregation, hypertension and vasospasm due to high cathecolamine levels (4).
Pheochromocytoma is a rare cause of hypertension and accounts for 0.5%to 2.0% of all causes of hypertension in children (5). Hypertension is one of the most common reasons of PRES.
The other common reasons for PRES are uremic encephalopathy, chronic renal disease, malignancy, chemotherapy agents, steroids therapy, vasculitis (6). Increased blood pressure is not an indispensable finding of PRES. The pathophysiology has not yet been well understood, but the main mechanism is considered as the endothelial dysfunction. It can be related with hypertension and leads to increased permeability in the blood-brain barrier (7). Endothelial toxicity due to circulating toxins or chemotherapy agents is other responsible factor (8, 9).
In PRES reversible hyperintense signal changes are seen on T2-weighted and FLAIR images due to vasogenic edema. The characteristic finding of PRES caused by hypertension is reversal of vasogenic edema after anti-hypertensive therapy. Hemorrhage, cytotoxic edema, and contrast enhancement are atypical imaging findings of PRES, which can be noted on severe cases (2). The most common and characteristic involvements occur in the cortical or subcortical white matter of the parietooccipital, frontal and temporal lobes (7, 10). However, the parieto-occipital cortex and subcortical white matter were preserved in our case. Lesions can be less frequently seen on brain stem, cerebellum, thalamus and basal ganglia. McKinney et al. (7) found that the involvement rate of brain stem is 18.4% and lentiform or caudate nuclei is 11.8%. Previous studies described that if there is brain stem or basal ganglia involvements with sparing parieto-occipital cortex and subcortical white matter, it is called central PRES (7). Central PRES was being reported in severe hypertensive cases which are similar to our case.
Basal ganglia and deep white matter involvement is especially seen in chronic hypertensive encephalopathy, because penetrating arteries are affected in chronic periods due to hyalin deposition within small arteries. In our case, vasogenic edema due to hypertensive encephalopaty was on bilateral caudate and lentiform nuclei, dentate nuclei and periventricular and deep white matter. Imaging findings indicated that the patient had chronical and untreated hypertension.
In previous studies; PRES was classified as mild, moderate, and severe (7, 11). Mild stage is identified as cortical and subcortical edema without parenchymal hemorrhage, herniation, mass effect and minimal or no involvement of cerebellum, brain stem, or basal ganglia. Moderate stage includes confluent edema extending from the cortex to the deep white matter without extension to the ventricular margin, or involvement of two groups of cerebellum, brain stem or basal ganglia. Mild mass effects and parenchymal hemorrhages can be seen, but there are no herniation or midline shifts. Severe stage is defined as confluent edema extending from the cortex to the ventricle, or hemorrhage with mass effects causing midline shift or herniation. Involvement of all 3 groups, the cerebellum, brain stem, and basal ganglia, is assessed as being severe. In our case, there was confluent edema extending from the cortex to the periventricular and deep white matter and edema in basal ganglia, dentate nuclei and multiple hemorrhagic foci in the basal ganglia, brain stem and periventricular and deep white matter, but no mass effects, shifts or herniation yet. Our findings were consistent with the severe PRES.
Susceptibility weighted imaging is a novel sequence used for detecting microhemorrhages, subarachnoid hemorrhage, deoxygenated blood, calcium and other metallic deposits (2) and is more sensitive than other conventional MRI sequences or CT scans for detecting microhemorrhages in the brain. McKinney et al. (2) found that the use of SWI improved the frequency for detections of hemorrhage, especially the microhemorrhage in PRES. In patients with chronic hypertension, multiple small infarctions and focal hemorrhages are irreversibly seen especially on the basal ganglia, thalamus and centrum semiovale (12). Since our patient had untreated and uncontrolled hypertension for seven years, we added SWI sequence in follow-up MRI to see if there are any microhemorrhagic foci in the brain in addition to macrohemorrhages. We observed that there were many michemorrhagic foci in the basal ganglia which are less frequently periventricular with a deep white matter and brain stem. Majority were not seen on the other MRI sequences. Our patient had already hypertensive related vasculopathy due to long standing uncontrolled hypertension since some changes especially in the periventricular region did not resolved in normotensive period. There were no genetic disease or connective tissue diseases affecting the vessels. This shows that perforating small vessels and associated parenchyma nearby ventricles were permanently affected due to long standing hypertensions.
In conclusion, intracerebral hemorrhage is a serious and unexpected complication of pheochromocytoma and is very rarely seen in childhood. Intracerebral hemorrhages should be investigated if there are hypertensive reversible signal changes within the brain. If the patient has a long history of uncontrolled severe hypertensions, we can expect additional hemorrhagic lesions in the brain. In case of chronic hypertension, if there are deeply located lesions such as within the basal ganglia or periventricular with deep white matter, the micro or macro hemorrhagic complications are also possibilities. The hemorrhage in basal ganglia is especially significant for chronic hypertensive encephalopathy. While vasogenic edema is reversible, the macro- and microhemorhages persist despite the anti-hypertensive therapy. SWI shows hemorrhagic complications of chronic hypertension since SWI is more sensitive to detect hemorrhages, as compared to other imaging methods.
Figures and Tables
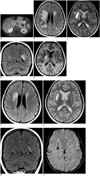 | Fig. 1MR appearance of hypertensive encephalopathy before treatment in patient with left surrenal pheochromocytoma.
A. Axial contrast enhanced T1-weighted MRI shows large heterogeneous enhancing mass with central cystic necrotic area on left adrenal gland (arrow). B-E. Axial FLAIR (B), T2-weighted (C), and coronal FLAIR (D) and T1-weighted (E) MRI images before anti-hypertensive therapy. Axial FLAIR image shows abnormal hyperintense signals indicating edema in periventricular and deep white matter and basal ganglia, more so, on right side (arrow) (B). Cerebral edema is observed as hyperintense signal on T2-weighted image in bilateral caudate and lentiform nuclei (black arrow) (C). Coronal FLAIR image shows hyperintense signal changes in dentate nuclei (arrows) (D). Also T2-weighted MRI shows two hyperintense lesions with peripheral hemosiderin rim; chronic hematoma is hypointense on T1-weighted images and hyperintense on T2-weighted images in right external capsule (white arrow), subacute hematoma in right globus pallidus is hyperintense on T1 and T2-weighted images (arrowhead) (E). FLAIR = fluid attenuated inversion recovery F-H. On tenth day of anti-hypertensive therapy; axial FLAIR image (F) demonstrates remarkable regression of high signal intensities in periventricular and deep white matter, axial T2-weighted (G) and coronal FLAIR (H) images show complete losses of high signal intensities in caudate, lentiform and dentate nuclei due to regressions of vasogenic edema. I. On susceptibility weighted imaging, there are millimetric signal losses in bilateral basal ganglia which are compatible with hemorrhagic foci. These were not seen on conventional MRI. FLAIR = fluid attenuated inversion recovery
|
References
1. Yeo H, Roman S. Pheochromocytoma and functional paraganglioma. Curr Opin Oncol. 2005; 17:13–18.
2. McKinney AM, Sarikaya B, Gustafson C, Truwit CL. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012; 33:896–903.
3. Singh VP, Singh M, Malhotra M, Kumar A, Agarwal AK. Extra-adrenal phaeochromocytoma-a case report of refractory hypertension. Indian Heart J. 2012; 64:203–205.
4. Eclavea A, Gagliardi JA, Jezior J, Burton B, Donahue JP. Phaeochromocytoma with central nervous system manifestations. Australas Radiol. 1997; 41:373–376.
5. Beltsevich DG, Kuznetsov NS, Kazaryan AM, Lysenko MA. Pheochromocytoma surgery: epidemiologic peculiarities in children. World J Surg. 2004; 28:592–596.
6. Kang E, Jeon SJ, Choi SS. Uremic encephalopathy with atypical magnetic resonance features on diffusion-weighted images. Korean J Radiol. 2012; 13:808–811.
7. McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007; 189:904–912.
8. Choi JM, Kim YH, Roh SY. Acute hepatic encephalopathy presenting as cortical laminar necrosis: case report. Korean J Radiol. 2013; 14:324–328.
9. Camara-Lemarroy CR, Lara-Campos JG, Perez-Contreras E, Rodríguez-Gutiérrez R, Galarza-Delgado DA. Takayasu's arteritis and posterior reversible encephalopathy syndrome: a case-based review. Clin Rheumatol. 2013; 32:409–415.
10. Jones BV, Egelhoff JC, Patterson RJ. Hypertensive encephalopathy in children. AJNR Am J Neuroradiol. 1997; 18:101–106.
11. Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol. 2000; 21:1199–1206.
12. Chester EM, Agamanolis DP, Banker BQ, Victor M. Hypertensive encephalopathy: a clinicopathologic study of 20 cases. Neurology. 1978; 28(9 Pt 1):928–939.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download