Abstract
We report the case of a 50-year-old woman presented with a history of right hemicolectomy due to an ileocecal neuroendocrine tumor and left breast metastasis. Owing to a slightly elevated chromogranin A-level and lower abdominal pain, single photon emission computed tomography-computer tomography (SPECT-CT) was performed. There were no signs of recurrence on the SPECT-CT scan, but the patient was incidentally found to have an inflamed intramural myoma. We believe that the slightly elevated chromogranin A-level was caused by the hypertension that the patient presented. In the clinical context, this is a report of an inflamed uterine myoma seen as a false positive result detected by TC-99m-Tc-EDDA/HYNIC-Tyr3-Octreotide (Tektrotyd) SPECT-CT hybrid imaging.
Increased expression of somatostatin receptors in the tumor cells of primary tumors, as well as in metastases, provides the molecular basis for the use of radio-labeled somatostatin analogues as part of diagnostic nuclear medicine (1, 2). The somatostatin analogue Tektrotyd is used to localize the neuroendocrine and non-neuroendocrine masses, which have a high density of somatostatin receptors (2).
In functional tomography diagnosis, single photon emission computed tomography (SPECT) provides information about the metabolic status of the tumor area. Exact anatomical mapping of focal findings is not always possible when using SPECT. In SPECT, activity distribution in the body is presented in the form of sectional images, similar to CT and magnetic resonance tomography. A CT provides detailed anatomical information about the region to be examined, yet often does not permit any specific diagnoses. In oncology patients, an image fusion is more diagnostically conclusive than merely comparisons of functional and morphological findings alone (3).
Clinical studies have provided evidence of uterine myoma in around 20% to 40% of all women over the age of 35. Uterine myomas are classified as intramural or subserosal myomas, according to their location. Intramural myomas are often asymptomatic and are localized in the myometrium. Pain only occurs in around 30% of women who have uterine myoma which are mostly a result of acute degeneration (4). These changes may arise when the blood supply to the myoma is not fully prevented, but merely impaired. Frequently, there is a tissue change, which results in a softer consistency of the uterine myoma. The formation of cysts containing gelatinous material is also typical. Inflammation and suppuration of a superficially located uterine myoma may cause severe complications, such as sepsis.
This case report presents an incidentally detected uterine myoma that had undergone inflammatory change in a patient with a neuroendocrine tumor; this scenario became apparent as the focal accumulation in the pelvis minor area was only shown in the course of a false positive SPECT-CT scan.
A 50-year-old patient was admitted to our hospital because of severe lower abdominal pain. Her history revealed a right hemicolectomy due to an ileocecal neuroendocrine tumor (NET). The histological findings revealed a well-differentiated NET with a solid, trabecular, gyriform, or glandular pattern, with fairly uniform nuclei, salt-and-pepper chromatin, and finely granular cytoplasm. Additionally, the patient had had a left breast amputation for metastasis of the NET.
Since blood tests revealed slightly elevated chromogranin A-levels, a SPECT-CT examination was carried out on a BrightView (Philips, Amsterdam, the Netherlands) system, a dual-head gamma-camera incorporating a low-dose, 6-slice, non-contrast-enhanced CT (12 mAs, 130 kVp, effective dose < 4 mSv) using TC-99m-Tektrotyd. Whole-body scans were performed 4 hours and 24 hours after intravenous administration of 569 MBq Tc-99m-Tektrotyd. The acquisition parameters for the SPECT included a 128 × 128 matrix, 3° angle step, and 120 frames per rotation. The CT scan duration was less than 1 minute. Overall, the SPECT-CT scan duration was about 30 minutes. CT and SPECT images were merged via a commercially available program (Image Volume Registration III, Philips, Amsterdam, the Netherlands) on to an Odyssey Workstation.
Single photon emission computed tomography revealed increased tracer retention in the pelvis; this could not be clearly anatomically assigned (Fig. 1A). After the image fusion, a focal accumulation was visible in the uterus (Fig. 1B, C). In addition to the SPECT-CT, we performed a coloscopy and a CT enteroclysis (Fig. 1D).
CT enteroclysis was performed with a 64-slice multidetector scanner (Brilliance 64, Philips Medical Systems, Cleveland, OH, USA) with intravenous injection of 120 mL of iohexol (Omnipaque®, GE Healthcare, Oslo, Norway) and an iodine concentration of 300 mgJ/mL. The rate of injection was set at 4 mL/s via an automatic power injector followed by a saline flush of 50 mL. The volumetric acquisition was performed in the late arterial dominant phase, 35 seconds after the start of injection. The CT enteroclysis scanning was performed in prone position, from the dome of the diaphragm to the pubic symphysis. The scan parameters were 64 × 0.625 mm collimation with a reconstruction interval of 0.89 mm, rotation time 0.75 s, tube voltage 120 kV, and 200 mAs. Reformats at 3 mm in the transverse, coronal, and sagittal planes were sent to the picture archiving system. Neither examinations showed signs of recurrence. Pelvic examination using intravaginal sonography (Fig. 1E), hysteroscopy, and laparoscopy yielded the diagnosis of an inflammatory intramural myoma, which was treated non-operatively. Histopathologic assessment revealed a myoma with presence of inflammatory cells. A cervical polyp was additionally diagnosed.
Somatostatin receptors (SSR) are membrane glycoproteins spreading over a large number body tissues and can be found in normal and pathological conditions (5).
Somatostatin receptor scintigraphy (SRS) detects neuroendocrine gastroenteropancreatic tumors with a high sensitivity (70% to 95%) (2, 6).
Gibril et al. (7) reported that, of all SRS examinations, 12% resulted in a false-positive localization for a neuroendocrine tumor or its metastases. Extra-abdominal false-positive localizations were more common than intra-abdominal. Thyroid disease, breast disease, and granulomatosis lung disease were the most frequent causes of extra-abdominal false-positive localizations. Accessory spleens, localization to previous operative sites, renal parapelvic cysts, and various procedural aspects were the most frequent causes of intra-abdominal false-positive localizations. SSR is over-expressed in activated peripheric lymphocytes and macrophages in granulomatous and inflammatory diseases (8).
Mena et al. (9) reported an incidental 111 pentetreotide uptake in a partially calcified uterine myoma. In our case, a focal accumulation was presumably caused by the expression of somatostatin receptors on the inflammatory cells of the myoma. Because of the negative results of the investigation described above, we think that the slight uptakes of chromogranin A was caused by the high blood pressure presented by the patient. As previously reported, high blood pressure can lead to an increase in the chromogranin A level in blood (10).
Compared to CT, somatostatin receptor scintigraphy has the advantage of being a whole-body investigation. Moreover, it can also guide treatments in the case of a positive scan (1). The use of SPECT-CT enables the accurate allocation of pathological and metabolically active nodules in anatomical regions. This case, involving a false positive SRS finding due to an inflammatory myoma, confirms that the use of SPECT and image fusion with CT leads to improvement in localization accuracy.
We would like to encourage physicians when performing Tektrotyd scans to be aware of inflammatory myoma as a cause of false positive results in a patient with lower abdominal pain.
Figures and Tables
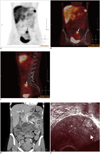 | Fig. 1SPECT (A), SPECT-CT (B, C), CT enteroclysis (D), intravaginal sonography of an inflammatory myoma.
A. Coronal image of SPECT scan performed 24 hours after administration of 569 MBq Tc-99m-Tektrotyd to evaluate possible NET demonstrated physiological uptake (liver, spleen, kidney, and bladder) and intense, ring-shaped uptake on left side of pelvis (long arrow). Short arrow in upper left abdomen shows physiological uptake by spleen. B, C. Fusion image of low-dose CT scan (coronal and sagittal images) performed in pelvic region, showing an uptake in anterior wall of uterus, consistent with intramural myoma. Study's findings show that Tektrotyd uptake is not specific for neuroendocrine tumors because somatostatin receptors (predominantly somatostatin receptor subtypes sst2 and sst5) are also over-expressed in different inflammatory cells, leading to visualization of sites of active inflammation and in endothelium. NET = neuroendocrine tumor, SPECT = single photon emission computed tomography D. There was no sign of recurrence on CT enteroclysis examination. Intramural myoma is illustrated in anterior wall of uterus (arrow). E. Transvaginal ultrasound shows intramural myoma in uterus (arrow).
|
References
1. Kölby L, Wängberg B, Ahlman H, Tisell LE, Fjälling M, Forssell-Aronsson E, et al. Somatostatin receptor subtypes, octreotide scintigraphy, and clinical response to octreotide treatment in patients with neuroendocrine tumors. World J Surg. 1998; 22:679–683.
2. Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993; 20:716–731.
3. Eubank WB, Mankoff DA, Schmiedl UP, Winter TC 3rd, Fisher ER, Olshen AB, et al. Imaging of oncologic patients: benefit of combined CT and FDG PET in the diagnosis of malignancy. AJR Am J Roentgenol. 1998; 171:1103–1110.
4. Walsh JW. Computed tomography of gynecologic neoplasms. Radiol Clin North Am. 1992; 30:817–830.
5. Kwekkeboom D, Krenning EP, de Jong M. Peptide receptor imaging and therapy. J Nucl Med. 2000; 41:1704–1713.
6. Joseph K, Stapp J, Reinecke J, Skamel HJ, Höffken H, Neuhaus C, et al. Receptor scintigraphy with 111In-pentetreotide for endocrine gastroenteropancreatic tumors. Horm Metab Res Suppl. 1993; 27:28–35.
7. Gibril F, Reynolds JC, Chen CC, Yu F, Goebel SU, Serrano J, et al. Specificity of somatostatin receptor scintigraphy: a prospective study and effects of false-positive localizations on management in patients with gastrinomas. J Nucl Med. 1999; 40:539–553.
8. Vanhagen PM, Krenning EP, Reubi JC, Kwekkeboom DJ, Bakker WH, Mulder AH, et al. Somatostatin analogue scintigraphy in granulomatous diseases. Eur J Nucl Med. 1994; 21:497–502.
9. Mena LM, Martłn F, Jiménez I, Ramos A. In-111 pentetreotide uptake in a uterine myoma. Clin Nucl Med. 2010; 35:524–525.
10. O'Connor DT. Plasma chromogranin A. Initial studies in human hypertension. Hypertension. 1985; 7(3 Pt 2):I76–I79.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download