INTRODUCTION
MATERIALS AND METHODS
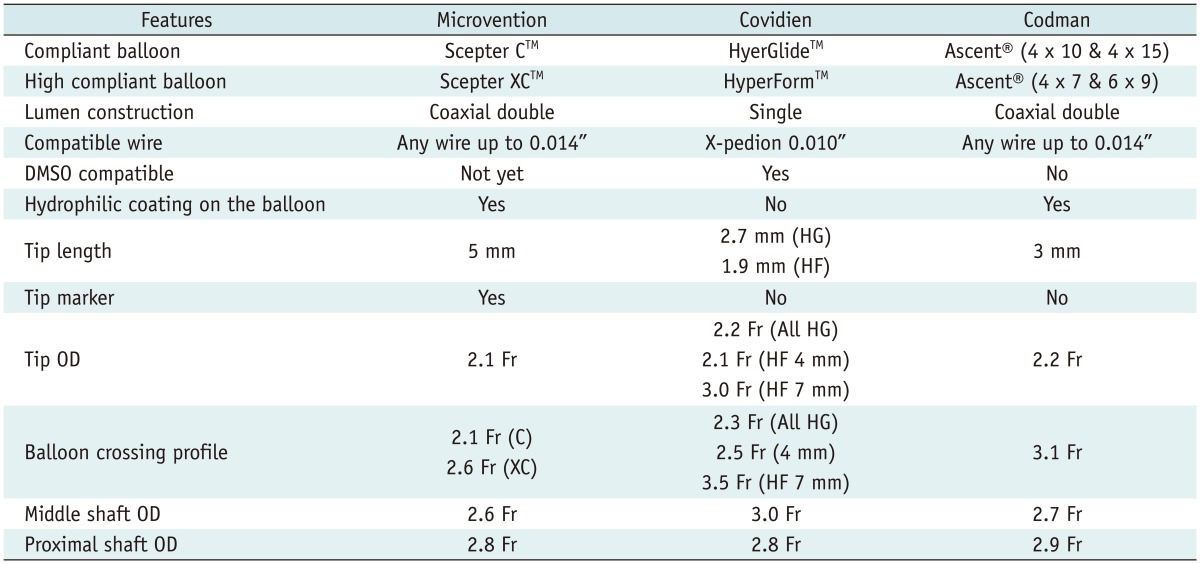
Scepter Balloon Catheter
Procedure
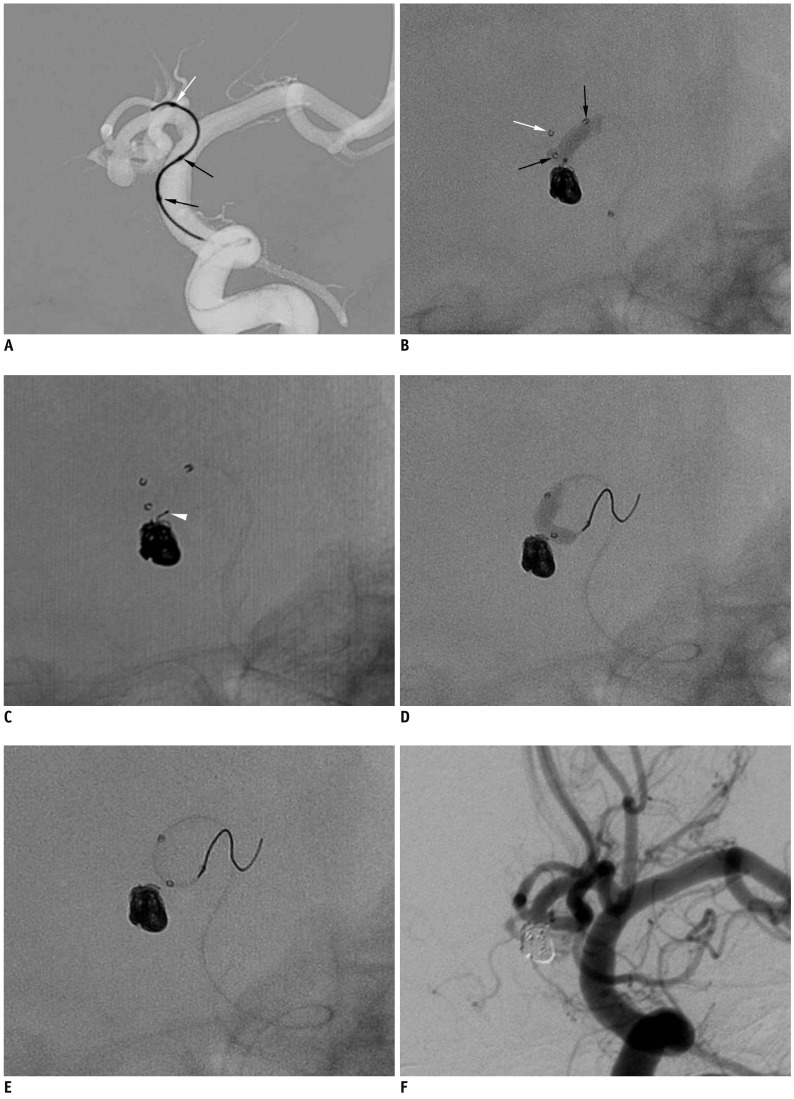 | Fig. 1Case 1. 72-year-old man with ruptured anterior communicating artery aneurysm.
A. Advancement of Scepter balloon catheter over 0.014-inch microguidewire that was prepositioned at anterior cerebral artery A1 portion. B. Scepter balloon-assisted coil embolization performed after removal of microguidewire. C. Coil tail (white arrowhead) protruded after balloon deflation. D, E. Scepter balloon catheter is repositioned from right A2 to left A2 using microguidewire and is used to push coil tail in sac. F. Final control angiogram shows complete occlusion of aneurysm sac without any coil loop protrusion. Note that white arrow indicates distal tip marker of Scepter balloon catheter and black arrows indicate proximal and distal markers of balloon itself.
|
RESULTS
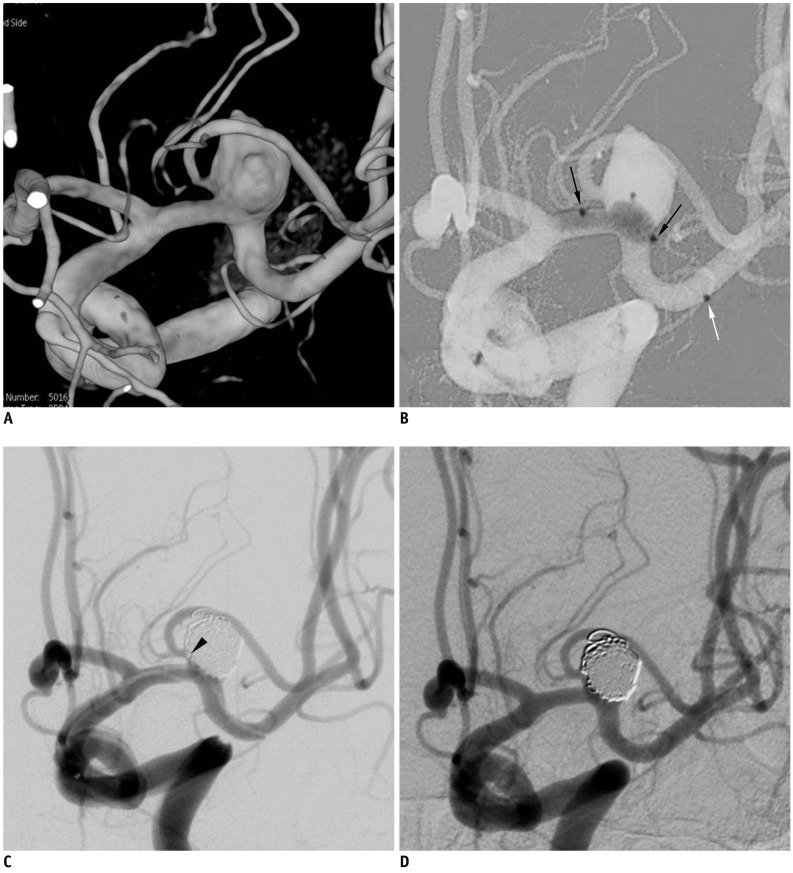 | Fig. 2Case 2. 66-year-old woman with ruptured aneurysm at middle left cerebral artery.
A. Three-dimension reconstruction angiogram reveals large aneurysm at middle left cerebral artery bifurcation. Note that superior branch is incorporated into sac. B. After placement of balloon across aneurysm neck, gradual over-inflation causes substantial portion of balloon to herniate into aneurysm sac. C. At end of procedure, small thrombus (black arrowhead) is detected at aneurysm neck, close to origin of superior branch. D. After intraarterial infusion of Glycoprotein inhibitor, 30-minutes follow-up angiogram shows resolution of thrombus and complete occlusion of aneurysm sac. Note that white arrow indicates distal tip marker of Scepter balloon catheter and black arrows indicate proximal and distal markers of balloon itself.
|
Table 2
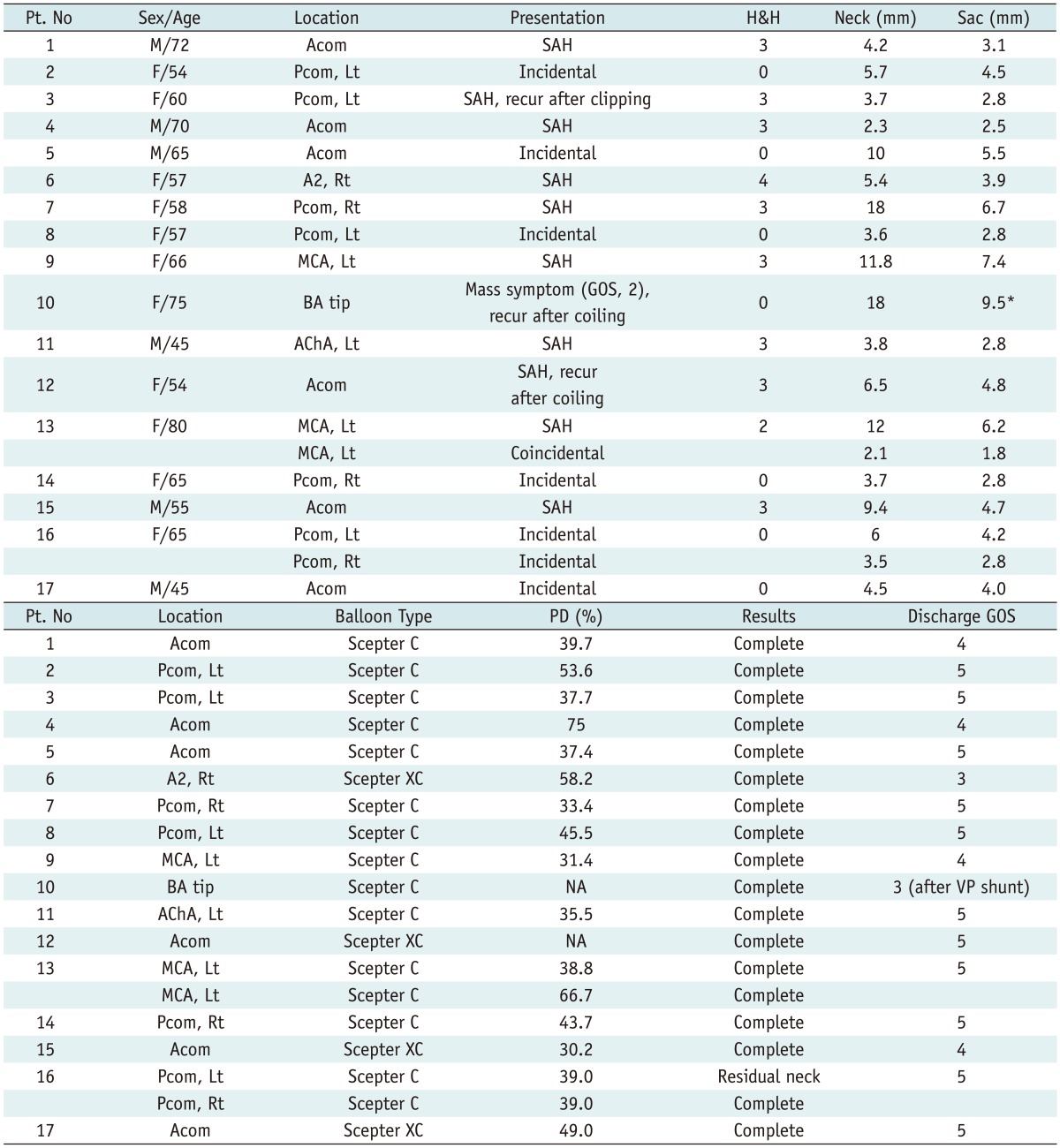
Note.- *Contrast medium filling portion, large portion of aneurysm was thrombosed. H&H = Hunt & Hess grade, Discharge GOS = glasgow outcome scale at discharge, PD = packing density, SAH = subarachnoid hemorrhage, Acom = anterior communicating artery, Pcom = posterior communicating artery, MCA = middle cerebral artery, BA = basilar artery, AChA = anterior choroidal artery, Lt = left, Rt = right, VP shunt = ventriculoperitoneal shunt
Representative Cases
Case 1 (of Patient No. 1)
Case 2 (of Patient No. 9)
Case 3 (of Patient No. 13)
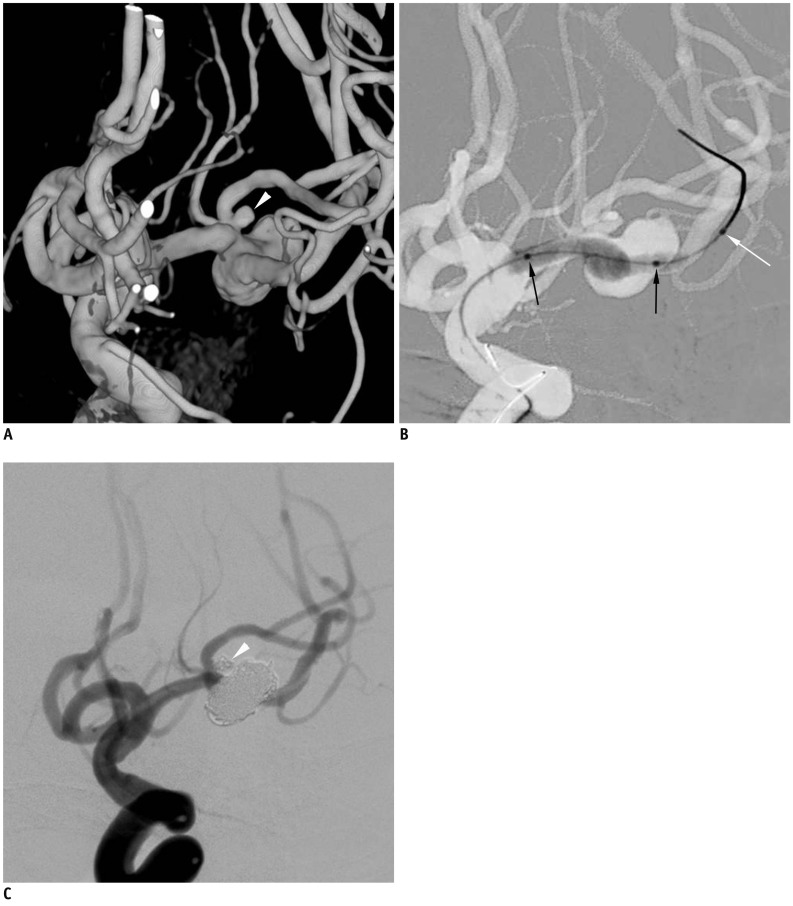 | Fig. 3Case 3. 80-year-old woman with ruptured middle left cerebral artery aneurysms.
A. Three-dimensional reconstruction angiogram reveals large aneurysm at middle left cerebral artery bifurcation and another very small aneurysm (white arrowhead) close to aneurysm neck at superior branch. B. After positioning Scepter balloon catheter across aneurysm neck, gradual over-inflation leads to herniation of central portion of balloon into large aneurysm sac. C. After balloon-assisted coil embolization of both aneurysms, final control angiogram shows complete occlusion of both aneurysms and well-preserved superior and inferior divisions. Black arrows indicate proximal and distal balloon markers of Scepter balloon catheter. White arrow indicated distal tip marker of Scepter balloon catheter (black arrows, proximal and distal balloon markers; white arrow, tip marker of balloon catheter).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download