Abstract
A 74-year-old man presented with a progressively worsening pain in sacrum and was diagnosed to have a sacral chordoma by biopsy in May, 2004. Percutaneous intratumoral injection with lipiodol-pingyangmycin suspension (LPS) was carried out under image guidance and repeated when the pain in sacrum recurred and the tumor increased. During a 6-year follow-up period, three sessions of this treatment were executed. CT imaging and Karnofsky Performance Score were used to evaluate the size of tumor and quality of life, respectively. The patient was free of pain after each procedure and had a high quality of life with a Karnofsky Performance Score above 80 points. The tumor lesion in sacral area was effectively controlled. No complications were observed. Percutaneous intratumoral injection with LPS under image guidance may be an effective and safe alternative for the patients with sacral chordoma.
Chordoma has poor sensitivity to radiotherapy and chemotherapy, and is mainly treated by surgery. However, chordoma still has a relatively high recurrence rate and a low disease-free survival rate after resection. Therefore, many authors suggest that patients with chordomas should be treated with a multidisciplinary team to obtain the optimal outcomes. We previously reported the safety and efficacy of percutaneous intratumoral injections with chemotherapeutic agent lipiodol suspension in treating the patients with hepatocellular carcinoma (1). Based on this, we utilized the method to treat patientx with sacral chordoma using lipiodol-pingyangmycin suspension (LPS). The following case report describes the results during a 6-year follow-up period.
A 74-year-old man presented with a progressively worsening pain in sacrum in January, 2004. MRI showed a mass lesion in the sacrum. In May, 2004, sacral pain was intolerable and accompanied with uroclepsia. Routine laboratory tests including blood routine, liver function and renal function all showed within normal limits. MRI showed the lesion increased in size and compressed the urinary bladder. The contrast-enhanced CT showed a 5-cm mass without enhancements (Fig. 1A). An additional puncture biopsy was confirmed for the histologic diagnosis of chordoma. The patient refused to undergo surgical resections of the tumor. Percutaneous intratumoral injection with LPS was therefore suggested as an alternative treatment. In China, the usage of LPS for the treatment of solid tumors had to be approved by Institutional Review Boards. The patient thus gave his written informed consent.
According to the CT images, the entry point of the skin was located on the left side of the 1st sacral vertebra with an 8-cm distance from the spine, and the direction of needle inserted into the tumor was a horizontal angle of 30°. The procedure was performed using a 15-cm-long, 21-gauge needle with a closed conical tip and three terminal side holes (Hakko, Tokyo, Japan) and under C-arm fluoroscopy (Axiom-Artis-dTA Angiographic System, Siemens Medical Systems, Germany) guidance.
The patient was put in prone position. Local anesthesia with 2% lidocaine was administered after local disinfections of the skin. The needle was inserted into the tumor under C-arm fluoroscopy, and the position of the needle tip was confirmed by anteroposterior and lateral fluoroscopy. Then, a 3 mL kermesinus liquid was aspirated from the tumor followed by injecting 3 mL of contrast medium (Iopamilon 300; Schering, Berlin, Germany) directly into the tumor. CT scan was immediately tested and showed that the contrast medium was accumulated within the tumor.
The procedure of intra-tumoral injection with LPS was performed afterwards. LPS was made as follows: pingyangmycin (PYM, Northern China Pharmaceutical Factory, Shijiazhuang, Hebei, China) 16 mg was dissolved in 5 mL of contrast medium and then mixed into a suspension with 5 mL lipiodol (LP, Laboratoire Guerbet, Roissy, France), and total LPS was about 10 mL in volume. Sequentially, LPS was manually injected slowly under simultaneous fluoroscopy until the vein drainage appeared. Then the needle was retracted 1-2 cm or adjusted to the direction of needling route. After the tip of needle within the lesion was confirmed by C-arm anteroposterior and lateral fluoroscopy, the additional injections were continued so that the LPS could be distributed throughout the entire tumor (Fig. 1B). Three days after the procedure, an X-ray radiography was taken and showed that the LPS deposited well within the sacrum tumor (Fig. 1C).
Follow-up CT scan showed that LP deposited well within the tumor 2 months after the first procedure. Surveillances of CT/MRI scans were required every 6 months thereafter. However, as a lack of compliance, the patient obtained follow-up CT scans again 28 months after the first procedure because of severe sacroiliac pains. It showed that the lesion obviously increased in size measuring 8.7 × 5.4 × 8.1 cm and LP thinly retained in the tumor (Fig. 1E). From September to October in 2006, the intratumoral injection with LPS was repeated in the same method 3 times under fluoroscopy guidance within the interval of one week, and the volume of LPS was respectively as follows: 20 mL (16 mg PYM, 10 mL LP, and 10 mL contrast medium), 10 mL (12 mg PYM, 5 mL LP, and 5 mL contrast medium), and 10 mL (12 mg PYM, 5 mL LP, and 5 mL contrast medium). LPS were distributed throughout the upper lesion after the first and second injections and the lower one after the third injection.
In April 2009, the patient was readmitted to our hospital, because he had experienced symptoms for more than one month, including severe sacroiliac pains, frequency and urgency of micturition, and weakness in lower limbs with greater severity on the right side. CT scan revealed that the sacrum tumor increased significantly and LP was deposited poorly in some regions. Intratumoral injection with LPS was repeated twice under CT guidance aimed to the region with only a little LP deposition in the interval of one week, with 20 ml of LPS (24 mg PYM, 10 mL LP, and 10 mL contrast medium) each time. In order to uniform the LPS distributions in the entire tumor mass, the third injection are required two weeks after being released from the hospital.
Routine laboratory tests were rechecked 3-7 days after the procedures, including blood routines, liver functions, and renal functions.
There were three sessions of treatments for the patient, and the intratumoral injection with LPS was repeated altogether 6 times with a total of 90 mL of LPS (PYM: 104 mg and LP: 45 mL) under fluoroscopy guidance (n = 4) and CT guidance (n = 2). The operation time ranged from 5 to 10 minutes (mean, 6.5 minutes) under fluoroscopy guidance, and it was 22 minutes and 31 minutes under CT guidance, respectively.
After the first procedure, the pain in sacrum gradually diminished. The patient demonstrated significant improvements in quality of life. CT scan showed that the tumor lesion decreased from 5 cm to 3 cm in diameter and the residual LP deposited well within the tumor 2 months after this procedure (Fig. 1D). Three days after the second procedure, CT scan showed that the tumor was filled fully with LPS (Fig. 1F). Symptoms were relieved and then vanished 3 weeks after the third procedure.
The patient did not experience adverse reactions after each procedure, such as fever, nausea, and vomiting. Nevertheless, 3-7 days after the procedure, his routine laboratory tests including blood routine, liver function and renal function showed within normal limits. There were only some LP depositions in gluteus after the second procedure without any discomfort (Fig. 1F). In the third procedure, disappointedly, the patient was not readmitted to the hospital to receive the third injection. No complications were observed during the follow-up period, such as skin necrosis, pelvic visceral injury, and neurological deficits.
In July, 2010, the patient presented with a severe pain in sacrum again. CT scan obtained in the local hospital revealed that the sacrum tumor increased significantly again, and lung metastasis appeared. The patient rejected further treatments and was unavailable for follow-ups. During a 6-year follow-up period, the patient demonstrated significant improvement in quality of life with a Karnofsky Performance Score above 80 points.
As we know, sacral chordoma is mainly treated by surgery. In order to improve therapeutic effects, some investigations have attempted to modify resections or combine with adjuvant treatments, such as radiotherapy, ablation, and embolization (2-6). Yang et al. (2) reported that surgical treatment combined with transcatheter arterial embolization could facilitate the removal of the sacral chordoma. Wagner et al. (3) reported that 5-year overall survival rates increased to 65% in the patients with surgical treatments combined with pre- and postoperative adjuvant radiotherapy. But surgery is difficult to perform in some cases due to the tumor location and size, patient comorbidities, and risk of complications (7). Minimal invasive alternatives have been increasingly used (8-11). Imai et al. (8) managed 95 patients with carbon ion radiotherapy, and the 5-year overall survival rate was 88%. However, two patients experienced severe skin necrosis and ultimately had to undergo skin grafts, and 15 patients experienced severe sciatic nerve complications requiring continuous medications. Percutaneous ethanol injection combined with radiotherapy has been used to treat patient with three postoperative recurrent tumors measuring 2-3 cm in diameter, and the tumors had been controlled for more than 6 years (9). Radiofrequency ablation (RFA) had been performed in two patients with postoperative recurrent tumor to relieve pains and improve the quality of life, but long-term therapeutic effects were not reported (10). Marchal et al. (11) also reported one patient treated with RFA, but he died of pseudoaneurysm rupture resulted from RFA. In addition, cryoablation had also been used to treat recurrent sacrococcygeal tumors in 5 patients, receiving local controls or palliations of pain at short-term follow-ups (12). To our knowledge, this article is the first clinical case describing percutaneous intratumoral injections with LPS for the treatment of sacral chordoma.
PYM, the single component of bleomycin A5, is an anticancer agent that is refined from Streptomyces pingyangensis and shows a strong damage function to tumor endothelial cells like bleomycin. It has been proven that the function depends on dosage and time according to our previous laboratory studies, especially in combination with LP, a high viscosity contrast medium (13, 14). As a drug-carrying agent with a high viscosity, we speculate LP accumulates in tumor lesions and cannot be easily drained with blood. LPS can make PYM release slowly. Prior to the treatment, the CT scan showed that the tumor lesion could not be enhanced, and the contrast medium, which was injected into the tumor lesion by percutaneous puncture, accumulated within for a considerable period. In our study, LPS was used as anti-tumor agents and administrated through the technique of percutaneous intratumoral injections but not the transcathter arterial infusion. Accordingly, in our previously clinical studies, we demonstrated that lipiodol-pingyangmycin was used to treat hemangioma and focal nodular hyperplasia of the liver in patients with their tumor diameters decreased significantly and without any complications (15, 16). The lipiodol-pingyangmycin sclerotherapy under fluoroscopic guidance was also considered as a safe and effective treatment for orbital venous malformations (17).
If we chose ethanol as injection agent, comparatively, it could directly induce tissue coagulation necrosis which results in preventing ethanol from wide diffusions. It is also necessary to account for the risk of important nerve injuries secondary to the leakage of ethanol. For this reason, percutaneous ethanol injections might be performed only in the patients with recurrent tumor, whose size is not large and relatively safe anatomic relationship (9). The PYM is a relatively safe drug for patients. PYM might cause some adverse reactions, but those are transient, including skin rash, nausea, vomiting, and fever. PYM might also lead to serious complications with pulmonary fibrosis in 30% patients when the amount of drug cumulates to reach 500 mg (18). But, in our study, we adopted a much lower dosage to locally inject from 12 to 24 mg per injection, reaching a total of 104 mg during a 6-year follow-up period, and such adverse reactions and serious complications did not occur.
During the procedure, it is the most important to make LPS uniformly distributed in the entire tumor lesion with less leakage into non-target areas. Thus, accurate and multidirection punctures and repetitive injections are common for each procedure, and injections must be performed under image guidance. We recommend the use of fluoroscopy as guidance tool due to the simultaneous observations for the distribution of LPS during injection. Although CT scan might be more propitious to guide the puncture, it must be repeatedly carried out to decrease the risk of LPS leakage. In our study, the CT scans were conducted to observe the distributions of LPS when each 2 mL of LPS was injected into the tumor.
Most surgeons agree that local recurrence is the most important determinant of long-term survival and that local control is the key to successful treatments: complete and radical resection contributes to high local control rates and the prolongation of disease-free survivals (19-21). In addition, the reported incidences of metastases range from 5 to > 65% in patients with long-term follow-ups (22). The incidence of metastases is more frequently in subtotal resection cases than complete resection cases (23). In our study, regular follow-up CT/MRI scan could not be performed to promptly detect the changes in tumor mass and LP depositions due to a lack of compliance. Local control achievements failed persistently without repeated injections to uniformly distribute LPS in the entire tumor lesion.
With the high recurrence rates of chordoma and incompleteness of LPS destroying tumor tissue, regular follow-ups and repetitious injections are necessary for the patients. Additionally, it may be feasible to improve the long-term therapeutic effects combined with other therapies (24), including surgery, transcatheter arterial embolization, and radiotherapy.
In conclusion, although experience remains limited, percutaneous intratumoral injection with LPS under image guidance may be an effective and safe alternative for the patients with sacral chordoma.
Figures and Tables
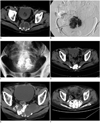
Fig. 1
Seventy four-year-old man with sacral chordoma treated by percutaneous LPS injection.
A. Contrast-enhanced CT showed 5-cm mass without enhancements in tumor. B. Injection was performed under fluoroscopy, and LPS distributed throughout entire tumor (Black arrow pointing to needle). C. LPS deposited well within sacrum tumor 3 days after first procedure. D. CT scan obtained 2 months after first procedure showed that tumor lesion decreased from 5 cm to 3 cm in diameter and residual LP deposited in tumor. LPS = lipiodol-pingyangmycin suspension. E. Twenty eight months (in Sep, 2006) after first procedure, follow-up CT scan showed that lesion obviously increased in size measuring 8.7 × 5.4 × 8.1 cm and LP thinly retained in tumor. F. 3 days after second procedure (in Oct, 2006), CT scan showed that tumor was fully filled with LPS and some LPS deposited in gluteus. LPS = lipiodol-pingyangmycin suspension
References
1. Chen Y, Li YH, Zeng QL, Zhao JB, Zhu QH, Huang DX, et al. Percutaneous intratumoral injection of lipiodol and chemotherapeutic agents emulsion for primary liver cancer. Chin J Gen Surg. 2009; 24:992–995.
2. Yang H, Zhu L, Ebraheim NA, Liu J, Shapiro A, Castillo S, et al. Surgical treatment of sacral chordomas combined with transcatheter arterial embolization. J Spinal Disord Tech. 2010; 23:47–52.
3. Wagner TD, Kobayashi W, Dean S, Goldberg SI, Kirsch DG, Suit HD, et al. Combination short-course preoperative irradiation, surgical resection, and reduced-field high-dose postoperative irradiation in the treatment of tumors involving the bone. Int J Radiat Oncol Biol Phys. 2009; 73:259–266.
4. Ruggieri P, Angelini A, Ussia G, Montalti M, Mercuri M. Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res. 2010; 468:2939–2947.
5. Osaka S, Kondoh O, Yoshida Y, Ryu J. Radical excision of malignant sacral tumors using a modified threadwire saw. J Surg Oncol. 2006; 93:312–317.
6. Baltsavias G, Valavanis A. Endovascular occlusion of a lacerated primitive trigeminal artery during surgical resection of clival chordoma. A case report. Interv Neuroradiol. 2010; 16:204–207.
7. Hsieh PC, Xu R, Sciubba DM, McGirt MJ, Nelson C, Witham TF, et al. Long-term clinical outcomes following en bloc resections for sacral chordomas and chondrosarcomas: a series of twenty consecutive patients. Spine (Phila Pa 1976). 2009; 34:2233–2239.
8. Imai R, Kamada T, Sugahara S, Tsuji H, Tsujii H. Carbon ion radiotherapy for sacral chordoma. Br J Radiol. 2011; 84 Spec No 1:S48–S54.
9. Nakajo M, Ohkubo K, Fukukura Y, Nandate T, Nakajo M. Treatment of recurrent chordomas by percutaneous ethanol injection therapy and radiation therapy. Acta Radiol. 2006; 47:297–300.
10. Anis N, Chawki N, Antoine K. Use of radio-frequency ablation for the palliative treatment of sacral chordoma. AJNR Am J Neuroradiol. 2004; 25:1589–1591.
11. Marchal F, Brunaud L, Bazin C, Boccacini H, Henrot P, Troufleau P, et al. Radiofrequency ablation in palliative supportive care: early clinical experience. Oncol Rep. 2006; 15:495–499.
12. Kurup AN, Woodrum DA, Morris JM, Atwell TD, Schmit GD, Welch TJ, et al. Cryoablation of recurrent sacrococcygeal tumors. J Vasc Interv Radiol. 2012; 23:1070–1075.
13. Zeng QL, Li YH, Chen Y, He XF, Lu Wei, Zhao JB, et al. The experimental study of intra-arterial infusion of pingyangmycin lipiodol emulsion in rabbits. J Clin Radiol (Chin). 2000; 19:376–379.
14. Kong WD, Li YH, Zeng QL, Chen Y, He XF. [Effect of pingyangmycin on the growth and cell cycle of human vascular endothelial cells]. Di Yi Jun Yi Da Xue Xue Bao. 2003; 23:830–832. 836
15. Zeng Q, Li Y, Chen Y, Ouyang Y, He X, Zhang H. Gigantic cavernous hemangioma of the liver treated by intra-arterial embolization with pingyangmycin-lipiodol emulsion: a multi-center study. Cardiovasc Intervent Radiol. 2004; 27:481–485.
16. Huang D, Chen Y, Zeng Q, Zhao J, Wu R, Wu X, et al. Transarterial embolization using pingyangmycin lipiodol emulsion and polyvinyl alcohol for the treatment of focal nodular hyperplasia of the liver. Hepatogastroenterology. 2011; 58:1736–1741.
17. Chen Y, Li Y, Zhu Q, Zeng Q, Zhao J, He X, et al. Fluoroscopic intralesional injection with pingyangmycin lipiodol emulsion for the treatment of orbital venous malformations. AJR Am J Roentgenol. 2008; 190:966–971.
18. Wu HB, Lu GC. Adverse reaction of pingyangmycin. Chin J Clin Pharm. 2001; 10:53–55.
19. Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000; 88:2122–2134.
20. York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999; 44:74–79. discussion 79-80.
21. Hanna SA, Aston WJ, Briggs TW, Cannon SR, Saifuddin A. Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008; 466:2217–2223.
22. Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC Jr. Lumbosacral chordoma. Prognostic factors and treatment. Spine (Phila Pa 1976). 1999; 24:1639–1645.
23. Yonemoto T, Tatezaki S, Takenouchi T, Ishii T, Satoh T, Moriya H. The surgical management of sacrococcygeal chordoma. Cancer. 1999; 85:878–883.
24. Hof H, Welzel T, Debus J. Effectiveness of cetuximab/gefitinib in the therapy of a sacral chordoma. Onkologie. 2006; 29:572–574.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download