Abstract
Objective
To describe the MRI findings in ten patients of spinal epidural angiolipoma for differentiated diagnosis presurgery.
Materials and Methods
Ten surgically proved cases of spinal epidural angiolipomas were retrospectively reviewed, and the lesion was classified according to the MR findings.
Results
Ten tumors were located in the superior (n = 4), middle (n = 2), or inferior (n = 4) thoracic level. The mass, with the spindle shape, was located in the posterior epidural space and extended parallel to the long axis of the spine. All lesions contained a fat and vascular element. The vascular content, correlating with the presence of hypointense regions on T1-weighted imaging (T1WI) and hyperintense signals on T2-weighted imaging, had marked enhancement. However, there were no flow void signs on MR images. All tumors were divided into two types based on the MR features. In type 1 (n = 3), the mass was predominantly composed of lipomatous tissue (> 50%) and contained only a few small angiomatous regions, which had a trabeculated or mottled appear. In type 2 (n = 7), the mass, however, was predominantly composed of vascular components (> 50%), which presented as large foci in the center of the mass.
Spinal epidural angiolipomas are rare tumors, accounting for 0.14% to 1.2% of spinal tumors and 20% to 22% percent of epidural tumors (1, 2). At present, MR imaging is one of the best imaging modalities of choice for assessing spinal angiolipomas (3). Approximately 128 cases of spinal epidural angiolipomas have been reported in literature to date (4). We retrospectively analyzed the MRI findings in ten patients of spinal epidural angiolipoma, which were confirmed pathologically, in order to investigate the value of MRI in diagnosis as well as the differential diagnosis of spinal epidural angiolipoma.
A review of our 12-year pathologic records (from January 1999 to December 2010) revealed 10 patients (5 males, 5 females; mean age, 50.8 years; range, 24 to 77 years) in whom histologic findings proved a diagnosis of spinal epidural angiolipoma.
The duration of symptoms varied from 6 days to 18 months. The main complaints were unilateral or bilateral lower extremity numbness or weakness progressively increasing. The clinical symptoms were sensory disorders (6 cases) or motor deficits (3 cases) and paralysis (1 case) below the level of the lesion. All ten cases were located in the thoracic spinal canal with no spinal congenital malformations (Table 1).
All ten cases had undergone an MRI examination, six cases scanned by GE Vectra 0.5 T superconducting MR machine, and the other four cases scanned by Marconi Eclipse 1.5 T superconducting MR machine. The sequences scanned included noncontrast T1-weighted (ten cases), T2-weighted (ten cases), noncontrast T2-weighted with fat saturation (five cases) and postcontrast T1-weighted (eight cases). The imaging parameters in T1-weighted imaging with SE sequence were as follows: 400-500/18-35/2 (repetition time/echo time/excitations). T2-weighted images were obtained using a fast spin-echo technique with parameters of 3800-4500/100-110/2. Both the T1-weighted and T2-weighted images were acquired with a section thickness of 3-5 mm with a 0.5-3.0 mm interslice gap and a matrix of 128 × 224 or 256 × 384. Fat-suppression T2-weighted images used short TI inversion recovery (STIR) with parameters of 1800/35/100/2 (repetition time/echo time/inversion time/excitations) and a matrix of 128 × 224 or 128 × 256. The contrast agent was gadolinium (Gd-DTPA) with a dose of 0.1 to 0.2 mmol/kg body weight.
Each case was evaluated independently by two radiologists, and a final diagnosis was reached by consensus. The lesion was assessed in the following categories: the entity of the tumor (including the location, shape, extent, signal features) and the change of the surrounding areas (including displacement or compression and signal change of spinal cord, widening of foraminal, bone erosion). The thoracic spine was divided into three part for the location as superior (T1-4), middle (T5-8) and inferior (T9-12). The craniocaudal extent of the lesion was measured compared to the vertebral body length. Then, the tumor was classified depending on the ratio of fat to vessels and the distribution of them.
Ten cases were located in the posterior epidural space of the thoracic spine, including four cases in the superior thoracic spine, two cases in the middle thoracic spine and four cases in the inferior thoracic spine.
The lesion of eight cases was spindle-shaped as a longitudinal axis parallel to that of the spine, both ends of which were spinous and pen-like (Fig. 1). The lesion of two cases extended into the adjacent intervertebral foramen with foraminal widening, and displayed as a dumbbell-shaped mass. All the cases showed an extradural mass, causing the displacement of the spinal cord without a conspicuous change in the signal, but no adjacent bone erosion.
The maximum craniocaudal extent of the lesion was about 5 vertebral body length and the minimum was approximately 1.5 vertebral body length with an average of 2.5 vertebral body length.
The normal dura displayed as a slit-like low signal on T2-weighted imaging (T2WI) between the tumor and the spinal cord. The ipsilateral subarachnoid space was narrowed and the spinal cord displaced ranged in all cases.
The MR signal of the tumor is composed of two parts: signal of the fat and blood vessels. The fatty content was hyperintense on both T1- and T2-weighted images (similar to the signal of subcutaneous adipose tissue) and hypointense on fat-suppressed images. The vascular component was hypointense on T1-weighted and hyperintense on T2-weighted, and showed intense enhancement with Gd-DTPA infusion. No case presented vascular flow voids on the MR images.
An intraspinal subacute hematoma was visualized in one case due to intratumoral bleeding. It also was isointense on T1-weighted and hypointense on T2-weighted in the central, but hypointense on T1-weighted and hyperintense on T2-weighted in the periphery (Fig. 2).
The epidural angiolipoma was divided into two types depending on the ratio of fat to vessels and the distribution of them.
Type 1: Three cases. The lesion was mainly demonstrated as a fatty signal (> 50%) scarred with thin-strips or spots of vessels. The signal of vessels could be clearly displayed on the fat-suppressed images, which were usually covered by the fatty signal on the conventional T1- and T2-weighted images (Fig. 1).
Type 2: Seven cases. The vascular component accounted for more than 1/2 of the lesion volume and was located in the central part of the tumor with the fat content surrounding it (Fig. 3).
All the patients underwent laminectomy. The epidural mass was poorly demarcated and easily bled with an aspect of grayish (type 1) or redness (type 2). Total removal was accomplished and decompression of the spinal cord was undertaken. The histopathologic examination revealed the tumor to be composed of mature adipose tissue and blood vessels (Fig. 2). All the patients were asymptomatic and recovered fully after surgery.
Spinal epidural angiolipoma was first descripted by Berenbruch in 1890 (5, 6) and introduced as an independent entity by Haddad et al. (7) in 1986. Since then, more than 100 cases of angiolipoma have been reported in the literature. All the ten cases in this report underwent an MR examination.
The pathogenesis of spinal epidural angiolipoma remains unclear. It once has been grouped as a variant of lipoma; however, now it can be distinguished from lipoma as there were several differences between them. First, the lipoma was primarily associated with spinal dysraphism (such as spina bifida) (6, 8), while the angiolipoma associated with spinal dysraphism has never been reported (9, 10). Second, the lipoma was usually seen in 20-year-old males, yet, it often occurred in females between the ages of 40 to 60 (11). Lastly, the lipoma was more commonly found in the lumbosacral rather than the thoracic (12). Haddad suggested that spinal epidural angiolipoma was a separate disease and therefore may arise from pluripotential mesenchymal cells which can differentiate into either fat or vessels (6, 7, 11). Hormone influences may also play a role in the generation of the tumor, as they occurred more during menopause or postmenopause.
The tumor was mostly located at the thoracic level (6, 10, 13). 90% of tumors were posterior in location (14, 15). The thoracic predominance could be interpreted by the spine's regional variation in the blood supply, as the thoracic spine is the least perfused (14). Our group of ten cases was all located in the thoracic.
Spinal epidural angiolipoma have a gross aspect of grayish-redness as well as a soft texture, encapsulated or uncapsulated, and can be easily dissected from the surrounding tissues (16). The spindle-shaped tumor is located in the spinal canal as a longitudinal axis parallel to that of the spine. A few cases of tumor grew along the intervertebral foramen and was presented as dumbbell-shaped (17). There were two cases in our group.
The tumor was composed of adipose and vascular elements. The ratio of fat to vessels range from 1 : 3 to 2 : 3 (7, 16). The fat components were the same as the normal adipose. The blood channels were composed as capillary, sinusoidal, thin-walled or thick-walled vessels with proliferation of the smooth muscle, and occasionally well-developed small artery (6, 18). The thin-walled blood vessels were mostly common seen and easily ruptured as the vessel wall was weak. In our group, one patient was demonstrated as an intraspinal hematoma due to intratumoral bleeding.
The clinical symptoms of spinal epidural angiolipoma were related to spinal cord compression, such as numbness, pain, weakness and sensory or motion changes below the level of lesion (6, 9, 15, 16, 19), often initiated as sensory abnormal of the feet and then progressively developing into lower extremity weakness or stiffness and finally, appear as sphincter dysfunction (1, 14, 18). The symptoms can be repetitioned due to venous thrombosis, blood deposition or blood flow diversions. Further, the symptoms deteriorated at times caused by acute intratumoral hemorrhage (6, 20). The symptoms of six cases in our group were presented as exacerbations, and one case was identified as sudden paraplegia due to intraspinal hematoma caused by acute intratumoral bleeding.
The MRI features of the epidural tumor: 1) A spindle-shaped tumor attached to the dura mater with both of the taper ends look like the tip of a pen in a sagittal aspect. 2) The ligament and dura mater displayed as a slit-like low signal between the tumor and spinal cord in T2WI. 3) The ipsilateral subarachnoid space narrowed, but the spinal cord shifted to the opposite side. All ten cases in our group displayed the above features and correctly disized the localization preoperatively.
A majority of tumors have typical MR features and can be diagnosed easily, as the signal of the lesion can be divided into two parts: the fatty content which was hyperintense on both T1-weighted and T2-weighted images and hypointense on fat-suppressed images (6, 13, 15, 16); the blood vessels showed to be hypointense in T1-weighted and hyperintense in T2-weighted with significant enhancement after the injection of Gd-DTPA. Blood flow-void shadow is rarely seen because the well-developed intratumoral small arterial are seldom seen (15, 19, 21). None of the ten cases showed a blood flow-void phenomenon. The vascular component could be well displayed in the fat suppression sequence. Extradural hematoma resulting from bleeding intratumoral can be detected. Acute hematoma manifested as isointense on T1-weighted and slightly hypointense on T2-weighted images, while the subacute phase of hematoma showed to be hyperintense on both T1-weigheted and T2-weighted images. The intense signal of the intraspinal subacute hematoma case in our group was complicated, which indicated repeated bleeding. It is not uncommon for tumors to grow along the intervertebral foramen outside the spinal canal; in particular, thoracic tumors can grow into the mediastinum formatting dumbbell-shaped mass. It can be misdiagnosed as mediastinal tumor when the intraspinal part is small, whereas the textraspinal lesion is relatively larger. Angiolipomas were usually thought to be benign tumors; however, some tumors demonstrated the invasion of the surrounding bone, which should be paid attention to (19, 22).
The differential diagnosis of epidural angiolipoma needs to consider the following directions according to the MRI classification. 1) It can be easily misdiagnosed as lipoma or lipomatosis of type 1 angiolipoma when the thin strip signals of the vascular component was covered by the signal of adipose tissue, particularly because the vascular components are rare. However, the lipoma or lipomatosis would not be expected to show a contrast enhancement (15). According to our experience, the fat suppression sequence is particularly important for this type of tumor, which is helpful in identifying small amounts of vascular components. The STIR technology we applied here is simple and relatively common. It depends on T1 which may affect the observation of the lesion because the T1 value of subacute hematoma was close to the adipose tissue in tumor hemorrhage. Therefore, some other adipose-tissue inhibition-specific sequence should be selected, such as chemical shift selective presaturation, in order to avoid this limitation. 2) The tumor of type 2 can be resembled as cavernous hemangioma or neurogenic tumors when the vascular components were abundant and concentrated in the central. One case was misdiagnosed as cavernous hemangioma in our report. There are slight differences between them. The limit between the entity of tumor and adipose tissue of cavernous hemangioma (or neurogenic tumor) were clear and slick as the surrounding adipose tissue is not a part of the tumor, but oppressed by the tumor (23), while the realm of vascular and adipose tissue of angiolipoma was vague and irregular. 3) Some epidural angiolipoma should be distinguished from mediastinal tumor when the intraspinal tumor is small but the textraspinal lesion is relatively larger. Further, it should be discriminated from epidural metastases when the adjacent bone is destructed by the tumor.
Hitherto, the MRI-typing in intraspinal epidural angiolipoma had never been reported in the English literature. We preliminary attempted to classify this tumor on the MRI based on the constituent ratio and distribution of them, to deepen the knowledge of imaging performance of the tumor and to simplify the process of differential diagnosis. However, the present study is limited by the study method of a retrospective review and a small number of cases, which did not allow us to summarized more correctly.
Figures and Tables
Fig. 1
Type 1 epidural angiolipoma. 50-year-old female with bilateral lower extremity adynamia of 18 months.
A. Noncontrast T1-weighted sagittal MR image showed homogeneous hyperintense mass compressing spinal cord and scarred with thin-strips or spots of vessels (arrow). Mass was spindle-shaped as longitudinal axis parallel to that of spine and both ends were spinous and pen-like. B. Noncontrast T2-weighted sagittal MR image showed that mass was also hyperintense. C. Postcontrast T1-weighted sagittal MR image showed obvious enhancement of mass. D. Postcontrast T1-weighted axial MR image showed mass displacing thecal sac anteriorly.

Fig. 2
Epidural angiolipoma with intratumoral hematoma. 24-year-old female with suddenly paraplegia of 6 days.
A. Noncontrast T1-weighted sagittal MR image showed epidural isointense mass with little hyperintense surrounding by. B. Noncontrast T2-weighted sagittal MR image showed that central of mass is hypointense but periphery is hyperintense. C. Fat-saturated T2-weighted sagittal MR image showed no decrease of hyperintense region. D. Noncontrast T1-weighted axial MR image showed mass slightly displacing thecal sac anteriorly. E. Microscopically, tumor was composed of abundant vascular channels (★) and mature adipose tissue (arrow), indicative of angiolipoma (haematoxylin and eosin stained, original magnification × 200).
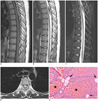
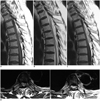
Fig. 3
Type 2 epidural angiolipoma. 77-year-old male with bilateral lower extremity numbness of 4 months.
A. Noncontrast T1-weighted sagittal MR image showed inhomogeneous mass that had component which demonstrated as being hypointense, while portions of tumor which were hyperintense were seen above and below hypointense area. B. Noncontrast T2-weighted sagittal MR image showed that mass which compressed spinal cord was hyperintense. Slit-like low signal could be seen between tumor and spinal. C. Postcontrast T1-weighted sagittal MR image showed inhomogeneous enhancement of mass without any sign of blood flow-void phenomenon. D. Postcontrast T1-weighted axial MR image showed mass locating in posterior epidural space of spinal.
References
1. Preul MC, Leblanc R, Tampieri D, Robitaille Y, Pokrupa R. Spinal angiolipomas. Report of three cases. J Neurosurg. 1993; 78:280–286.
2. Samdani AF, Garonzik IM, Jallo G, Eberhart CG, Zahos P. Spinal angiolipoma: case report and review of the literature. Acta Neurochir (Wien). 2004; 146:299–302. discussion 302.
3. Nanassis K, Tsitsopoulos P, Marinopoulos D, Mintelis A, Tsitsopoulos P. Lumbar spinal epidural angiolipoma. J Clin Neurosci. 2008; 15:460–463.
4. Ghanta RK, Koti K, Dandamudi S. Spinal epidural angiolipoma: a rare cause of spinal cord compression. J Neurosci Rural Pract. 2012; 3:341–343.
5. Berenbruch K. Ein Fall von multiple Angio-lipomen kombiniert mit einem Angiom des Ruckenmarks. Tibingen: 1890.
6. Fourney DR, Tong KA, Macaulay RJ, Griebel RW. Spinal angiolipoma. Can J Neurol Sci. 2001; 28:82–88.
7. Haddad FS, Abla A, Allam CK. Extradural spinal angiolipoma. Surg Neurol. 1986; 26:473–486.
8. Walsh JW, Markesbery WR. Histological features of congenital lipomas of the lower spinal canal. J Neurosurg. 1980; 52:564–569.
9. Thomas JE, Miller RH. Lipomatous tumors of the spinal canal. A study of their clinical range. Mayo Clin Proc. 1973; 48:393–340.
10. Shibata Y, Sugimoto K, Matsuki T, Nose T. Thoracic epidural angiolipoma--case report. Neurol Med Chir (Tokyo). 1993; 33:316–319.
11. Petrella G, Tamburrini G, Lauriola L, Di Rocco C. Spinal epidural angiolipoma complicated by an intratumoral abscess. Case report. J Neurosurg. 2005; 103:2 Suppl. 166–169.
12. Shuangshoti S, Hongsaprabhas C. Intraspinal epidural angiolipoma. J Med Assoc Thai. 1979; 62:457–460.
13. Poon TP, Behbahani M, Matoso IM, Katz MA, Pearl M. Epidural angiolipoma with spinal cord compression. J Natl Med Assoc. 1988; 80:347–349.
14. Hungs M, Paré LS. Spinal angiolipoma: case report and literature review. J Spinal Cord Med. 2008; 31:315–318.
15. Provenzale JM, McLendon RE. Spinal angiolipomas: MR features. AJNR Am J Neuroradiol. 1996; 17:713–719.
16. Gelabert-González M, García-Allut A. Spinal extradural angiolipoma: report of two cases and review of the literature. Eur Spine J. 2009; 18:324–335.
17. Sakaida H, Waga S, Kojima T, Kubo Y, Matsubara T, Yamamoto J. Thoracic spinal angiomyolipoma with extracanal extension to the thoracic cavity. A case report. Spine (Phila Pa 1976). 1998; 23:391–394.
18. Turgut M. Spinal angiolipomas: report of a case and review of the cases published since the discovery of the tumour in 1890. Br J Neurosurg. 1999; 13:30–40.
19. Leu NH, Chen CY, Shy CG, Lu CY, Wu CS, Chen DC, et al. MR imaging of an infiltrating spinal epidural angiolipoma. AJNR Am J Neuroradiol. 2003; 24:1008–1011.
20. Akhaddar A, Albouzidi A, Elmostarchid B, Gazzaz M, Boucetta M. Sudden onset of paraplegia caused by hemorrhagic spinal epidural angiolipoma. A case report. Eur Spine J. 2008; 17:Suppl 2. S296–S298.
21. Weill A, del Carpio-O'Donovan R, Tampieri D, Melanson D, Ethier R. Spinal angiolipomas: CT and MR aspects. J Comput Assist Tomogr. 1991; 15:83–85.
22. Kujas M, Lopes M, Lalam TF, Fohanno D, Poirier J. Infiltrating extradural spinal angiolipoma. Clin Neuropathol. 1999; 18:93–98.
23. Zhong W, Huang S, Chen H, Sun H, Cai B, Liu Y, et al. Pure spinal epidural cavernous hemangioma. Acta Neurochir (Wien). 2012; 154:739–745.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download