Abstract
We report in a 65-year-old man hepatocellular carcinoma adjacent to a transjugular intrahepatic portosystemic shunt stent-graft which was successfully treated with irreversible electroporation (IRE). IRE is a new non-thermal tissue ablation technique which uses electrical pulses to induce cell necrosis by irreversible membrane poration. IRE proved to be more advantageous in the ablation of perivascular tumor with little injury to the surrounding structures.
Radiofrequency ablation (RFA) is a well-established minimally invasive tumor therapy, which is based on the thermal effects that cause tumor necrosis (1). However, issues associated with perfusion-mediated tissue cooling or heating are still a significant challenge with thermal ablation techniques. The ablation of lesions close or adjacent to larger blood vessels is hindered by the heatsink effect (2). Furthermore, liver ablations close to the adjacent organs (e.g., gallbladder, stomach, bowel and heart) are either risky or cannot be performed at all. In contrast to thermal ablation techniques, irreversible electroporation (IRE) is a new non-thermal ablation technique that - instead of heat - uses electric impulses to cause an irreversible disruption of cell membrane integrity, resulting in cell death in the ablated region (3). We report and describe the first non-thermal IRE ablation of a hepatocellular carcinoma (HCC) adjacent to a transjugular intrahepatic portosystemic shunt (TIPS) stent-graft, where thermal ablation could not be performed.
A 65-year-old-patient with alcoholic liver cirrhosis was admitted for inpatient care due to a liver lesion that was detected during a routine ultrasound examination. Using contrast-enhanced ultrasound examination (CEUS), we found a HCC suspicious lesion with arterial hyperperfusion and increased wash-out during the portalvenous phase. In order to check the suspected diagnosis of HCC, we performed a contrast enhanced MRI scan and furthermore, a biopsy was taken. Both measures confirmed the diagnosis of HCC (2.2 × 4.0 cm) adjacent to a TIPS stent-graft in segment IVa/V which was implanted in January 2006 due to portal hypertension and therapy for refractory variceal bleeding. Due to the patient's general bad state of health (Karnofsky performance status scale 20%) and impaired liver function test (Child-Pugh Score B), he could not undergo surgical therapy. RFA in the vicinity of the TIPS stent-graft was ruled out because of the risk of incomplete ablation due to the heat-sink effect as well as the risk of TIPS membrane destruction and thus potentially occluding the stent lumen. A superselecive transarterial chemoembolization procedure was deemed to be the best treatment option. However, during liver angiography, multiple pronounced arteriovenous shunts were depicted (Fig. 1A). After careful consideration of the remaining treatment options (systemic therapy with sorafenib, non-thermal ablation with percutaneous ethanol injection [PEI]), we decided to perform a percutaneous IRE ablation of the lesion. Informed consent was obtained.
The patient was put under general anesthesia and neuromuscular blocking using atracurium, cisatracurium, pancuronium or equivalent in order to prevent arrhythmia (5). The procedure was performed using the NanoKnife® system (Angiodynamics®, Latham, NY, USA) with the following system parameters, as recommended by the manufacturer: number of electrodes: 3; distance of electrodes: min. 0.7 cm, max. 2.0 cm; voltage: min. 1050.0 kV, max. 3000.0 kV; pulse length: 90 µsec; impulses per electrode per cycle: 70. The percutaneous placement of the electrodes was guided by CT-fluoroscopy (Fig. 1B) as well as an ultrasound using a multi-frequency probe (1-5 MHz, Logiq E9/GE, Fairfield, CT, USA) with Doppler imaging and CEUS. For CEUS bolus injection of 2.4 mL microbubbles of sulfohexafluoride (SonoVue®, Bracco, Milano, Italy) was used. A total of three electrodes were placed into the tumor volume in a triangular shape. To cover the complete tumor lesion, the electrodes had to be repositioned four times in order to ablate all the viable tumor cells. During the IRE procedure, the patient did not have any cardiovascular events; in particular, there were no supraventricular tachycardia and no atrial fibrillation. Complications, particularly pneumothorax, were not observed. After the ablation procedure, the patient did not complain about upper abdominal pain or shortness of breath. The patient's 24 hour follow-up imaging examinations as well as followup imaging after 8 weeks (consisting of CEUS, MRI and a CT scan) showed complete ablation of the lesion (Fig. 1C-F). The color coded Doppler sonography showed a normal function of the TIPS after the ablation (150 cm/s systolic flow velocity, 800 mL/min volume flow). Despite the ablation of a centrally located lesion, the follow-up laboratory and sonography studies the following day and three months after ablation proved that the patient did not get cholestasis (bilirubin in normal limit; 1.1 mg/dL).
Image guided percutaneous tumor ablation has gained high acceptance in the treatment of malignant liver lesions when surgical options are precluded (7). Consequently, according to the Barcelona Clinic Liver Cancer, staging classification and treatment schedule for percutaneous ablation is recommended in patients with early-stage hepatocellular carcinoma, if patients are excluded from surgical options (11). Among the different ablation techniques, thermal ablation with radiofrequency is the most widespread technique and has been shown to be associated with higher efficiency and greater survival benefit compared to the non-thermal PEI (8, 12). However, centrally located or subcapsular lesions close to the gallbladder, stomach, bowel or heart still pose a challenge for percutaneous thermal ablation techniques in the liver. Due to the spread of heat to the adjacent structures, there is a risk of thermal damage. Several methods have been proposed to overcome the heat mediated destruction of adjacent structures, such as instillation of air, CO2 or 5% dextrose water (9, 10). Nevertheless, there are still locations, e.g., subcapsular lesions close to the heart, where thermal protection of the adjacent structures is not possible. The ablation of centrally located liver lesions is hindered by the heatsink effect as well as the risk of damage to the central bile ducts (13). Thus, there are still circumstances where a percutaneous ablation of malignant liver lesions is either risky or cannot be performed at all.
Irreversible electroporation, as new non-thermal ablation technique of soft tissue, offers the possibility to overcome the aforementioned limitations of thermal ablations. Two or more monopolar probes or a single bipolar probe must be used at a time. The number of monopolar probes that are used during an IRE procedure depends on the size and shape of the desired zone of tissue ablation. The system can be used with up to six single electrode probes at a time. The treatment parameter for voltage depends on the distance between the probes within the targeted tissue. IRE uses a series of electrical pulses for microseconds in order to generate irreversible permeabilization of cell membranes, and thereby induces apoptosis in the treated tissue. IRE proved to be highly effective in tissues with high density of cell wall structures and less effective in tissues with high concentration of collagenous and elastic fibers (4). As a non-thermal technique, IRE is not limited by the heatsink effect. There appears to be complete ablation up to the margin of blood vessels without compromising to the functionality of the blood vessels (6). This - in contrast to thermal ablation - allows tumor cell ablation without concomitant destruction of the connective tissue, bile ducts, blood vessels and nerves, which implies the ablation of tumor cells also in those areas where thermal ablation was not possible before.
Irreversible electroporation proved to be a promising treatment option in patients with inoperable HCC. In central tumor lesions close to the liver hilum or lesions close to larger blood vessels and bile ducts, conventional RFA cannot be performed. Furthermore, subcapsular lesions and those in the vicinity of other organs (i.e., heart, gallbladder, stomach and bowel) are not treatable with conventional RFA. Due to its selective and non-thermic ablation and the obvious decrease of limitations and contraindications, IRE widens the field of minimally invasively treatable lesions.
As a first step, we demonstrated in our case that a tissue around a TIPS stent-graft can be fully ablated by IRE with complete preservation of the stent-graft and the TIPS flow. We also found no impairment of bile flow even though a large centrally located tissue volume was ablated. For further evaluation of the IRE ablation technique regarding the extent of ablation, the procedure's side effects and patients' outcome as well as the potential overall-survival benefits, prospective studies will have to be performed.
Figures and Tables
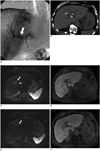 | Fig. 1Pre-interventional angiography and intra-procedural CT scan and pre- and post-ablation MR images.
A. Angiographic image during TACE. Celiac arteriography shows diffuse arteriovenous shunts (white arrow). TACE failed due to shunt. B. CT image during CT fluoroscopy-guided IRE. Precontrast CT shows low-attenuation ablation defect abutting TIPS stent graft (arrows). Note deployment of electrodes adjacent to TIPS stent graft. TACE = transarterial chemoembolization, TIPS = transjugular intrahepatic portosystemic shunt. C-F. Pre- and post-ablation MR images. C. Preablation diffusion-weighted MR Image (b = 1000 sec/mm2) shows slightly hyperintense HCC (white arrow) adjacent to TIPS stent graft (white arrowhead). D. Preablation T1-weighted image during hepatobiliary phase shows hypointense HCC which measures 2.2 × 4.0 cm (black arrow). E. 8-week postablation diffusion-weighted MR-image (b = 1000 sec/mm2) shows complete ablation of HCC (arrow). F. 8-week postablation T1-weighted MR-image shows hypointense sharp demarcation of ablation zone. HCC = hepatocellular carcinoma, TIPS = transjugular intrahepatic portosystemic shunt
|
References
1. Yun BL, Lee JM, Baek JH, Kim SH, Lee JY, Han JK, et al. Radiofrequency ablation for treating liver metastases from a non-colorectal origin. Korean J Radiol. 2011; 12:579–587.
2. Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998; 227:559–565.
3. Dupuy DE, Aswad B, Ng T. Irreversible electroporation in a Swine lung model. Cardiovasc Intervent Radiol. 2011; 34:391–395.
4. Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007; 6:287–294.
5. Deodhar A, Dickfeld T, Single GW, Hamilton WC Jr, Thornton RH, Sofocleous CT, et al. Irreversible electroporation near the heart: ventricular arrhythmias can be prevented with ECG synchronization. AJR Am J Roentgenol. 2011; 196:W330–W335.
6. Kingham TP, Karkar AM, D'Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg. 2012; 215:379–387.
7. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.
8. Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009; 49:453–459.
9. Kamusella P, Wiggermann P, Wissgott C, Andresen R, Stroszczynski C. [Thermal protection of targeted air instillation in CT-guided radiofrequency ablation]. Rofo. 2011; 183:952–955.
10. Raman SS, Lu DS, Vodopich DJ, Sayre J, Lassman C. Minimizing diaphragmatic injury during radio-frequency ablation: efficacy of subphrenic peritoneal saline injection in a porcine model. Radiology. 2002; 222:819–823.
11. Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012; 262:43–58.
12. Chang NK, Shin SS, Kim JW, Kim HJ, Jeong YY, Heo SH, et al. Effect of ultrasound-guided radiofrequency ablation in incompletely treated hepatocellular carcinoma after transcatheter arterial chemoembolization. Korean J Radiol. 2012; 13:Suppl 1. S104–S111.
13. Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012; 13:34–43.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download