Abstract
Objective
To assess the clinical efficacy, safety, and risk factors influencing local tumor progression, following CT-guided radiofrequency ablation (RFA) of recurrent or residual hepatocellular carcinoma (HCC), around iodized oil retention.
Materials and Methods
Sixty-four patients (M : F = 51 : 13, 65.0 ± 8.2 years old) with recurrent or residual HCC (75 index tumors, size = 14.0 ± 4.6 mm) had been treated by CT-guided RFA, using retained iodized oil as markers for targeting. The technical success, technique effectiveness rate and complications of RFA were then assessed. On pre-ablative and immediate follow-up CT after RFA, we evaluated the size of enhancing index tumors and iodized oil retention, presence of abutting vessels, completeness of ablation of iodized oil retention, and the presence of ablative margins greater than 5 mm. Also, the time interval between transarterial chemoembolization and RFA was assessed. The cumulative local tumor progression rate was calculated using the Kaplan-Meier method, and the Cox proportional hazards model was adopted, to clarify the independent factors affecting local tumor progression.
Results
The technical success and technique effectiveness rate was 100% and 98.7%, respectively. Major complications were observed in 5.6%. The cumulative rates of local tumor progression at 1 and 2 years were 17.5% and 37.5%, respectively. In multivariate analyses, partial ablation of the targeted iodized oil retention was the sole independent predictor of a higher local tumor progression rate.
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide, and most deaths occur within one year of diagnosis (1). Radiofrequency ablation (RFA) is widely accepted as an effective treatment for patients with early-stage HCC (2). Several observational studies and randomized control trials have demonstrated that RFA is comparable to hepatic resection, with regard to the overall survival of patients with early-stage HCC (3-5). Computed tomography (CT) is one of the most common guiding tools for the RFA of HCC. CT guidance has advantages over ultrasound (US) guidance, in cases where the index tumors are not identifiable by US examination, such as tumors that are located in the hepatic dome, which are typically difficult to observe using US (6). Furthermore, in patients who previously underwent transarterial chemoembolization (TACE), residual or recurrent marginal tumors are sometimes difficult to differentiate in US images from areas with compact iodized oil retention (7). Several studies have reported satisfactory efficiency and a low complication rate with CT-guided RFA (8-11).
When RFA is performed under CT guidance, iodized oil retained from the prior TACE is commonly used as a targeting marker. The images used for CT guidance are non-contrast, and they do not visualize enhanced arterial index tumors well; thus, the iodized oil remaining from a previous therapy is useful as a targeting marker (12). Furthermore, the effectiveness of a combined therapy consisting of various treatment options, including TACE, RFA, percutaneous ethanol injection and radiation therapy (13-15), has recently been reported. Therefore, cases that implement RFA immediately following TACE, as part of a combined therapy plan, are becoming more common. In these cases, retained iodized oil can be an effective marker for CT guidance.
To our knowledge, however, technical efficacy, safety, and the risk factors influencing local tumor progression after CT-guided RFA of recurrent or residual HCC around retained iodized oil have not been reported. Therefore, the purpose of this study was to assess the therapeutic efficacy, safety and risk factors for local tumor progression of CT-guided RFA, using iodized oil retentions as markers for targeting.
This study was approved by the institutional review board; however, written informed consent was waived, because of the retrospective nature of the study. All procedures related to this study were performed after written consent was obtained.
For the 66-month period between March 2005 and August 2010, a total of 476 consecutive sessions of RFAs were performed using US or CT guidance in our institutes. Among them, 95 sessions of CT-guided RFAs were performed. Two sessions were excluded, because they involved metastatic tumors other than HCC. Fifteen sessions of CT-guided RFA were performed in cases without iodized targeting oil abutting index tumors, and were therefore excluded. Seven sessions were also excluded, because index tumors did not show typical imaging features (arterial enhancement followed by delayed washout on dynamic contrast enhanced CT) for the diagnosis of HCC, which was proposed by American Association for the Study of Liver Disease (AASLD) (2). The remaining 71 RFA sessions, in which the retained iodized oil was used as an anatomic landmark to place an electrode, were included in the study. These 71 CT-guided RFA sessions were performed in 64 patients (M : F = 51 : 13, mean age = 65.0 ± 8.2 years and age range = 48-80 years) for 75 index tumors. Demographic findings of the patients are summarized in Table 1. For all index tumors, the diagnosis of HCC was based on the guidelines of the AASLD, and pathological confirmation was not obtained in any index tumor (2).
All the patients had already undergone TACE with a mixture of doxorubicin hydrochloride (ADM; Dong-a Pharmacy, Seoul, Korea), iodized oil (Lipiodol; Guerbet, Cedex, France), and absorbable gelatin sponge particles (Gelfoam; Baxter, Hayward, CA, USA). In all patients, however, residual or recurred, enhancing index tumors were noted abutting the iodized oil retentions. Five interventional radiologists, each of whom had more than five years of experience in TACE, have participated in the TACE procedures during the study period, and they used the same techniques. The median time interval between TACE and CT-guided RFA was 8 weeks (range: two days to 40 months).
The pre-RFA CT scans were performed within four weeks of the RFA treatment. Multi-detector CT (MDCT) examinations were performed using a LightSpeed Ultra 16-detector row scanner (GE Healthcare, Milwaukee, WI, USA), or a LightSpeed VCT (GE Healthcare, Milwaukee, WI, USA). Unenhanced, arterial phase, portal venous phase and equilibrium phase images were acquired. For contrast enhancement, a total of 120 mL of iopromide (Ultravist 300; Bayer Schering Pharma, Berlin, Germany) was injected, at a rate of 3-4 mL/sec.
The pre-RFA CT scans were retrospectively assessed by two abdominal radiologists working in consensus, both of whom had more than five years of experience interpreting oncologic images. The CT evaluations included the size of the index tumors and the targeted iodized oil area, and the presence of blood vessels close to the tumors. Blood vessels close to the index tumors were defined as blood vessels larger than the third order branches of the hepatic or portal veins abutting the index tumors.
The RFA procedures and data assessment methods used in this study were based on the standardized terms and reporting criteria proposed by the Society of Interventional Radiology (16). The CT-guided RFA was performed using a four-detector MDCT scanner (MX8000; Phillip Medical Systems, Best, the Netherlands). The procedures were performed by one interventional radiologist who had five years of experience in CT-guided tumor ablation. The RFA procedures employed at our institution were similar to those described by several authors (8-11). Analgesia was achieved by intravenous administration of 50-100 µg of fentanyl citrate (Fentanyl Citrate GuJu INJ; Guju Pharma, Seoul, Korea), and local injection of 5-15 mL of 1% lidocaine (Lidocaine HCl Daihan INJ; DaiHan Pharmaceutical Co., Seoul, Korea). Plastic grids were applied to the skin, and pre-treatment CT imaging of the liver was then performed, to determine the optimal skin entry point, and the safest route of electrode insertion. The electrode was then inserted towards the area of iodized oil retention. We used internally cooled, 17-gauge, 15- or 20-cm-long, single RF electrodes with a 3-cm-long exposed metallic tip (Cool-tip; Radionics, Burlington, MA, USA, or Well Point RF electrode; STARmed, Goyang, Korea), and a 500-kHz monopolar RF generator (CC-1; Radionics, Burlington, MA, USA) capable of producing 200 W of power. The RF energy was delivered in the impedance control mode for 10 to 27 minutes, depending on the size and number of index tumors.
During the ablation therapy, our goal was to achieve complete ablation of the index tumor, and to create an ablative margin of at least 0.5 cm of normal liver parenchyma. Also, we tried to ablate the iodized oil retentions themselves, if technically available. A smaller ablated zone could occur around iodized oil retention, and therefore we tried to locate electrodes in the appropriate area, and, if necessary, multiple overlapping applications were performed. Multiple ablation techniques were also adopted in the treatment of the larger index tumors of more than 2 cm in diameter. A 0.5-cm ablative margin was adopted on the basis of previous reports that a 0.5-cm to 0.3-cm ablative margin is associated with a lower rate of local tumor progression, after RFA of HCC (17-19). Additionally, we recorded whether a transpulmonary route was used to introduce the electrode.
Follow-up CT scans were obtained immediately after the RFA, to detect any residual tumors, and observe any immediate complications. To evaluate the technique effectiveness rate, the patients underwent a CT exam, one month after the RFA procedure. Subsequent follow-up CT scans were repeated every three months. The follow-up CT scans were performed in the same way as the pre-RFA CT scans.
The follow-up CT scans were retrospectively assessed by two abdominal radiologists working in consensus. The scans were evaluated for the completeness of index tumor ablation, presence of ablative margins greater than 5 mm, local tumor progression, and complications. We also noted whether the targeted iodized oil retention areas were completely ablated. This concept of complete ablation of the targeted iodized oil retention areas is explained in Figure 1. Figures 2, 3 illustrate representative cases of complete and partial ablation of iodized oil retention areas, respectively.
Technical success was defined as the complete coverage of enhancing, index tumors by ablated area, with no residual marginal enhancement, on immediately acquired CT scans following the RFA, regardless of coverage of iodized oil retentions by the ablated area. The technique effectiveness rate was defined as the percentage of index tumors that were successfully eradicated one month after the RFA (16). An ablative margin was defined as a region ablated beyond the border of the tumor. Ablative margins were measured based on axial images, by a previously reported method (19). Complications were classified as major or minor, according to the definitions of the Society of Interventional Radiology (16, 20). Local tumor progression was diagnosed, when the follow-up CT data showed interval tumor development or growth within 1 cm of the margin of the ablation zone or targeted iodized oil retention areas. Tumor development or growth was diagnosed by the presence of marginally enhancing nodular tumors with typical imaging features of HCC, or growing hypovascular tumors of low attenuation, on serial follow-up CT scans.
Technically successful and effectively treated index tumors were analyzed for factors influencing local tumor progression. Cumulative local tumor progression rates were calculated using the Kaplan-Meier method. The Cox proportional hazards model was used to assess the independent predictors for local tumor progression, while adjusting for confounding variables. Cumulative local tumor progression rates were calculated for the independent predictors, and then compared, using the log-rank test. All the statistical analyses were performed using commercially available software (STATA 10.0 for Windows; Stata Corp LP, College Station, TX, USA), and p-values less than 0.05 were considered statistically significant.
Table 2 shows the characteristics of the 75 ablated tumors. 90.7% of the index tumor were smaller than 20 mm, and the mean tumor size was 14.0 ± 4.6 mm (range = 10-37 mm). The iodized oil retention area was smaller than 20 mm in 88.0%, and the mean size was 12.0 ± 9.8 mm (range = 2-63 mm). Complete ablation of the iodized oil retention areas occurred in 76.0% of index tumors.
The technical success rate was 100.0% (75/75 index tumors). The technique effectiveness rate at one month after the RFA was 98.7% (74/75 tumor nodules). Small residual tumors were found around one index tumor, in the follow-up CT images taken one month after RFA. The follow-up periods ranged from 1 to 68 months after the RFA (mean = 19.5 ± 16.2 months).
The major and minor complications are summarized in Table 3. Minor complications occurred in 23 sessions (32.4%). Major complications were noted in four sessions (5.6%), and no complication led to mortality. In one patient, a large diaphragmatic defect was created, and a transdiaphragmatic hernia of the right colon was noted 15 months later; a right hemicolectomy was required for bowel necrosis (Fig. 4). Pleura-related complications, including pneumothorax, pleural effusion, hydropneumothorax and a diaphragm defect, developed in 22 patients. In all of these 22 patients, the RF electrode was inserted via a transpulmonary route. The incidence of pleura-related complications during the transpulmonary approach was 61.1% (22/36 sessions), though 81.8% of them (18/22 complications) were minor complications.
The cumulative rates of local tumor progression at 1 and 2 years were 17.5% and 37.5%, respectively. Table 4 shows the results of the multivariate analyses, using the Cox proportional hazards model. A multivariate analysis showed that the completeness of the ablation of the areas of iodized targeting oil retention (hazard ratio 5.073, range 1.690-15.230) was the sole significant independent factor of local tumor progression. Figure 5 shows the difference of the curves for local tumor progression patterns between the groups of complete and partial ablation of iodized oil retention, with p-values calculated using the log-rank test. The two groups showed significant different cumulative local tumor progression rates: 13.8% versus 29.1% at 1 year, and 22.4% vs. 79.0% at 2 years, respectively (p < 0.001).
Ultrasound is the most commonly implemented guidance tool for RFA, and high technical efficacy and safety was reported in cases of residual or recurrent HCC after TACE (21). However, US targeting is not always possible with tumors that are not easily visualized using US. Rhim et al. (22) showed that approximately 30% of small HCCs referred for possible percutaneous RFA were not conspicuous on US, and were thus excluded from US-guided RFA. Several strategies have been developed, to attempt to solve these problems. First, an US contrast agent and artificial ascites or pleural effusion has been used, to enhance the visibility of tumors in US images (23, 24). A fusion of CT and US images has also been utilized for navigation, though this system is not always available in many medical institutes (25, 26).
Previous studies have examined the technical efficacy and safety of CT-guided RFA for HCC (8-11). The technical success in these studies has been reported to be 87.3-97%, with major complication rates and minor complication rates of 0-7.7% and 2.8-33%, respectively. No mortality has been reported. Because these published results for CT-guided RFA are similar to those found in our study, CT-guided RFA can also be considered to be a safe and effective procedure, in cases of residual or recurrent HCC around retained iodized oil after TACE. In this study, the pleural-related complications, such as pleural effusion, pneumothorax and hydropneumothorax, occurred in 61.1% of the cases treated using the transpulmonary approach. Of the transpulmonary cases, 11.1% resulted in major complications. Although it was difficult to determine a precise cause in these situations, it is plausible that the retraction of the electrodes during the ablation may have caused a diaphragm injury that was more severe than expected. On the other hand, when using US-guidance, pleura-related complications were less frequently reported, in cases of the RFA of HCC abutting diaphragm (27). Higher incidence of pleura-related complications in CT-guided RFA might be caused by the fact that the transpulmonary approach is inevitable for index tumors located in the hepatic dome, when CT-guidance is adopted for the RFA. Therefore, a transpulmonary approach should be performed with extra caution.
The local tumor progression rate after RFA has been reported to be 2.4-12%, and the known risk factors for local tumor progression include large tumor size, insufficient ablative margins, the presence of adjacent large blood vessels, a poor histological grade, and the degree of arterial enhancement of the index tumors (19, 28-32). In this study, multivariate analyses confirmed that the incomplete ablation of the iodized oil retention areas as a sole independent predictor of local tumor progression, but the relationships between local tumor progression and the previously known risk factors listed above revealed unexpected results. These results may be attributable to the following factors: 1) the average tumor size was small; 2) all the index tumors enrolled in this study showed arterial enhancement; and 3) the histological grade analysis was not available. A large tumor size and an insufficient ablative margin have a reciprocal relationship with each other, and may be correlated with the presence of adjacent large vessels. In this study, however, the index tumor size was smaller than 2 cm in 89.2% of the cases; consequently, the ablative margin was also sufficient, which might be the cause of the non-significance of tumor size and ablative margins in multivariate analyses.
This study confirmed that incomplete ablation of the targeted iodized oil retention area affects local tumor progression. This finding will not be surprising to many interventional radiologists. Many assume that the oil distribution is the same as the tumor distribution, and already attempt to ablate iodized oil retention areas. However, it has been reported that 75.1% of iodized oil retentions smaller than 5 cm in diameter and treated by super-selective TACE showed total necrosis of the tumor in pathologic examination (33). Additionally, if a radiologic response longer than 6 months revealed no marginal recurrence of iodized oil retention areas, 91.3% of the tumors showed total necrosis (34). Therefore, for cases in which complete ablation of iodized oil retention area is made difficult by various reasons (larger size of iodized oil retention, abutting vessels, difficult location, etc.), the necessity of ablating the entire iodized oil retention area might be thrown into question for interventional radiologists. This study resolved that question, by proving the importance of complete ablation of targeted iodized oil retention areas. Iodized oil retention can be thought of as the egg yolk, and the surrounding ablative zone as the egg white; the treatment should aim to create a post-ablative lesion in the form of a sunny-side-up fried egg.
Fluoroscopy guidance can also be used in cases of recurrent or residual HCC around retained iodized oil. Recently, Min et al. (35) reported the results RFA of viable HCC around retained iodized oil, after TACE using biplane fluoroscopy plus US guidance. They reported only 9.5% of local tumor progression for a mean follow-up period of 20.3 months, and no major complication. These results are better than ours, and the causes of these good results are: first, dual guidance of the fluoroscopy and US with realtime monitoring; second, less frequent transpulmonary approaches. Fluoroscopic guidance is also superior to CT, in terms of radiation exposure to both the patient and operator. However, small iodized oil retention could not be visualized by fluoroscopy, and most of the iodized oil retentions of our study were smaller than 2 cm. Therefore, we believe CT guidance still has its role in the RFA of recurrent or residual HCC around iodized oil retention.
Our study has several limitations. First, a pathological confirmation of HCC was not available in all cases. Additionally, the diagnosis of local tumor progression was not based on pathological examination. However, we made an effort to follow the AASLD clinical guidelines for diagnosing HCC. Therefore, we believe that the presence of typical imaging features most likely indicates a correct positive diagnosis. Second, most of the tumors were smaller than 2 cm in diameter, and showed arterial enhancement, and these findings may have influenced the multivariate analyses. Third, retention of iodized oil diminishes over time, and the time interval between TACE and RFA is important. In addition, if the iodized oil retention area is followed for a long time, without evidence of recurrence, there might be little chance of another local tumor progression (34). However, in this study, the time interval was too wide (from two days to 42 weeks). Even though we included a time interval between TACE and RFA for multivariate analyses, the wide range of the time interval may influence the results of this study. Fourth, iodized oil retention also depends on HCC tumor etiology and biology. In this study, the index tumors were predominantly small (< 2 cm in 89.2%), and secondary to maternally transmitted Hepatitis B virus (67.6%). These findings may not be generalized to other patient populations.
In conclusion, CT-guided RFA of HCC using iodized oil retention as a targeting marker was effective and safe. The incomplete ablation of retained iodized oil was an independent predictor of local tumor progression. Therefore, local tumor progression can be minimized, by complete ablation of both the index tumors, and the areas of iodized oil retention.
Figures and Tables
Fig. 1
Schematic diagrams illustrating complete and partial ablation of area of iodized oil retention for targeting.
A. Complete ablation of area of iodized oil retention for targeting. Ablated area (dashed circle) completely covers marginal local tumor progression/residual tumor (black circle) and iodized oil retention area (white circle) that were used as target for CT-guided RFA. B. Partial ablation of area of iodized oil retention for targeting. Ablated area (dashed circle) completely covers local tumor progression/residual tumor (black circle). However, iodized oil retention area (white circle), used as target for CT-guided RFA, is not completely covered by ablated area. RFA = radiofrequency ablation
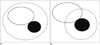
Fig. 2
Complete ablation of enhancing tumor, and iodized oil retention, in 60 year-old man with multinodular hepatocellular carcinoma.
A. On late arterial phase images of CT scan acquired before RFA, there is enhancing, nodular tumor, with partial retention of iodized oil in right hemiliver dome area (arrow). There are small iodized oil retentions in liver parenchyma. B. CT-guided RFA was performed (arrow). Radiofrequency electrode punctured center of iodized oil retention. C. On portal venous phase images of CT scan acquired immediately after RFA, enhancing tumor and areas of iodized oil retention are completely covered by low attenuation ablated area (arrow). In this patient, no local tumor progression was observed, during follow-up period of 24 months. RFA = radiofrequency ablation
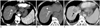
Fig. 3
Complete ablation of marginal recurred tumor, but partial ablation of iodized oil retention, in 58 year-old man with multinodular hepatocellular carcinoma.
A. In late arterial phase images of CT scan acquired before RFA, there is enhancing nodular tumor, abutting area of iodized oil retention in right anterior section of liver (arrow). B. CT-guided RFA was performed (arrowheads). Radiofrequency electrode punctured anterior margin of iodized oil retention area. C. In portal venous phase images of CT scan acquired immediately after RFA, enhancing tumor is completely covered by ablated area. However, area of iodized oil retention is not completely covered by low-attenuation ablated area. D. After four months, local tumor progression was observed in posterior region of iodized oil retention area (arrow), which was not covered by ablated area. RFA = radiofrequency ablation

Fig. 4
Diaphragm defect and herniated colon as major complication, in 68 year-old woman with single nodular hepatocellular carcinoma.
A. There is enhancing index tumor in S8 of cirrhotic liver (arrow). Minor iodized oil retention is noted in posteromedial region of tumor. Significant ascites is also observable. B. CT-guided RFA was performed through transpulmonary route, and targeted using iodized oil retention as marker. C. CT image three months after D. There is large defect of diaphragm (arrow heads), and herniated right colon in right hemithorax (arrows). Right hemicolectomy was required in this patient, due to ischemic change in right hemicolon. RFA = radiofrequency ablation

Fig. 5
Kaplan-Meier curves, showing significant differences in local tumor progression rate. Local tumor progression rate was significantly better in cases of complete ablation of targeted iodized oil retention areas, than cases of partial ablation (complete ablation = solid line, partial ablation = dotted line). P-values are calculated by log-rank test.
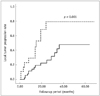
Table 2
Tumor and Ablation Characteristics of 75 Index Tumors Treated by CT-Guided Radiofrequency Ablations Using Iodized Oil Retentions for Targeting

Table 3
Complications Associated with CT-Guided Radiofrequency Ablation Using Iodized Oil Retention for Targeting

References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
2. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.
3. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006; 243:321–328.
4. Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010; 51:1284–1290.
5. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008; 47:82–89.
6. Lee MW, Kim YJ, Park HS, Yu NC, Jung SI, Ko SY, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010; 194:W396–W400.
7. Min JH, Lee MW, Rhim H, Choi D, Kim YS, Kim YJ, et al. Recurrent hepatocellular carcinoma after transcatheter arterial chemoembolization: planning sonography for radio frequency ablation. J Ultrasound Med. 2011; 30:617–624.
8. Kim YK, Kim CS, Lee JM, Chung GH, Chon SB. Efficacy and safety of radiofrequency ablation of hepatocellular carcinoma in the hepatic dome with the CT-guided extrathoracic transhepatic approach. Eur J Radiol. 2006; 60:100–107.
9. Laspas F, Sotiropoulou E, Mylona S, Manataki A, Tsagouli P, Tsangaridou I, et al. Computed tomography-guided radiofrequency ablation of hepatocellular carcinoma: treatment efficacy and complications. J Gastrointestin Liver Dis. 2009; 18:323–328.
10. Park BJ, Byun JH, Jin YH, Won HJ, Shin YM, Kim KW, et al. CT-guided radiofrequency ablation for hepatocellular carcinomas that were undetectable at US: therapeutic effectiveness and safety. J Vasc Interv Radiol. 2009; 20:490–499.
11. Toyoda M, Kakizaki S, Horiuchi K, Katakai K, Sohara N, Sato K, et al. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol. 2006; 12:608–611.
12. Laganà D, Carrafiello G, Mangini M, Lumia D, Mocciardini L, Chini C, et al. Hepatic radiofrequency under CT-fluoroscopy guidance. Radiol Med. 2008; 113:87–100.
13. Takaki H, Yamakado K, Uraki J, Nakatsuka A, Fuke H, Yamamoto N, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas larger than 5 cm. J Vasc Interv Radiol. 2009; 20:217–224.
14. Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. 2006; 16:661–669.
15. Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008; 247:260–266.
16. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009; 20:7 Suppl. S377–S390.
17. Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010; 195:758–765.
18. Liu CH, Arellano RS, Uppot RN, Samir AE, Gervais DA, Mueller PR. Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol. 2010; 20:877–885.
19. Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007; 188:480–488.
20. Burke DR, Lewis CA, Cardella JF, Citron SJ, Drooz AT, Haskal ZJ, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. Society of Cardiovascular and Interventional Radiology. J Vasc Interv Radiol. 1997; 8:677–681.
21. Chang NK, Shin SS, Kim JW, Kim HJ, Jeong YY, Heo SH, et al. Effect of ultrasound-guided radiofrequency ablation in incompletely treated hepatocellular carcinoma after transcatheter arterial chemoembolization. Korean J Radiol. 2012; 13:Suppl 1. S104–S111.
22. Rhim H, Lee MH, Kim YS, Choi D, Lee WJ, Lim HK. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. AJR Am J Roentgenol. 2008; 190:1324–1330.
23. Miyamoto N, Hiramatsu K, Tsuchiya K, Sato Y, Terae S, Shirato H. Sonazoid-enhanced sonography for guiding radiofrequency ablation for hepatocellular carcinoma: better tumor visualization by Kupffer-phase imaging and vascular-phase imaging after reinjection. Jpn J Radiol. 2009; 27:185–193.
24. Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008; 190:91–98.
25. Lee MW, Rhim H, Cha DI, Kim YJ, Choi D, Kim YS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol. 2012; 198:1438–1444.
26. Minami Y, Chung H, Kudo M, Kitai S, Takahashi S, Inoue T, et al. Radiofrequency ablation of hepatocellular carcinoma: value of virtual CT sonography with magnetic navigation. AJR Am J Roentgenol. 2008; 190:W335–W341.
27. Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009; 10:34–42.
28. Cho YK, Rhim H, Noh S. Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: a systematic review. J Gastroenterol Hepatol. 2011; 26:1354–1360.
29. Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008; 207:20–29.
30. Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003; 14:1267–1274.
31. Park Y, Kim YS, Rhim H, Lim HK, Choi D, Lee WJ. Arterial enhancement of hepatocellular carcinoma before radiofrequency ablation as a predictor of postablation local tumor progression. AJR Am J Roentgenol. 2009; 193:757–763.
32. Waki K, Aikata H, Katamura Y, Kawaoka T, Takaki S, Hiramatsu A, et al. Percutaneous radiofrequency ablation as first-line treatment for small hepatocellular carcinoma: results and prognostic factors on long-term follow up. J Gastroenterol Hepatol. 2010; 25:597–604.
33. Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011; 53:1580–1589.
34. Kim SJ, Choi MS, Kang JY, Choi DI, Park CK, Gwak GY, et al. Prediction of complete necrosis of hepatocellular carcinoma treated with transarterial chemoembolization prior to liver transplantation. Gut Liver. 2009; 3:285–291.
35. Min JH, Lee MW, Rhim H, Choi D, Kim YS, Kim YJ, et al. Radiofrequency ablation for viable hepatocellular carcinoma around retained iodized oil after transcatheter arterial chemoembolization: usefulness of biplane fluoroscopy plus ultrasound guidance. Korean J Radiol. 2012; 13:784–794.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download