Abstract
Post-transplantation lymphoproliferative disorders (PTLDs) are a heterogeneous group of diseases that represent serious complications following immunosuppressive therapy for solid organ or hematopoietic-cell recipients. In contrast to B-cell PTLD, T-cell PTLD is less frequent and is not usually associated with Epstein Barr Virus infection. Moreover, to our knowledge, isolated T-cell PTLD involving the breast is extremely rare and this condition has never been reported previously in the literature. Herein, we report a rare case of isolated T-cell PTLD of the breast that occurred after a patient had been treated for allogeneic peripheral blood stem cell transplantation due to acute myeloblastic leukemia.
Post-transplantation lymphoproliferative disorders (PTLDs) are a heterogeneous group of diseases that represent serious complications following immunosuppressive therapy in solid organ or hematopoietic cell recipients (1-8). PTLD shows a spectrum of unregulated lymphoid expansion ranging from polyclonal hyperplasia to monoclonal malignant lymphoma, which usually involves lymph nodes and extra-nodal solid organs of the abdomen, thorax, head and neck, and brain (3-8).
To our knowledge, PTLD presenting as an isolated T-cell lymphoma involving the breast is extremely rare. In this report, we describe the imaging findings and pathologic correlation of PTLD presenting as an isolated peripheral T-cell lymphoma of the breast in a 22-year-old woman, who had received allogenic peripheral blood stem cell transplantation (allo-PBSCT).
A 22-year-old woman, who had received allo-PBSCT due to acute myeloblastic leukemia, came to the oncology department for a regular check-up. Except for a recently developed cough, the patient had no B symptoms (i.e., fever, weight loss, night sweats). She had undergone computed tomography examinations of the chest and abdomen, in which exhibited no abnormal findings suggestive of relapse. In addition, the patient complained of a vaguely palpable mass in her right breast, and therefore, she had undergone whole breast ultrasonography (US) examination to evaluate the lesion.
Ultrasonography examination revealed 2 suspicious hypoechoic masses showing ill-defined margins, and mixed echo pattern with hyperechoic boundaries, and an area of focal parenchymal bulging in the right breast (Fig. 1A-D). Mammography performed thereafter revealed 3, ill-defined, isodense masses in the right breast, which corresponded to the lesions detected on breast US (Fig. 1E, F). US-guided core biopsy was performed targeting at the suspicious breast mass, and histologic examinations showed diffuse infiltration of large lymphoid cells with pleomorphic, irregular and prominent nuclei, and frequent mitoses that include some atypia (Fig. 1G). Immunohistochemistry staining demonstrated that the tumor cells were positive for CD3, CD45, bcl-2, and focal positivity for CD5, but were negative for CD21, CD30, CD56 (Fig. 1H). Epstein Barr Virus (EBV) in situ hybridization using Epstein-Barr-encoded RNA showed a negative reaction among the tumor cells. Final pathologic diagnosis of the breast mass was monomorphic peripheral T-cell, post-transplantation lymphoma. For further staging and evaluation of the presence of PTLD involvement of other organs, Fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG PET-CT) was performed. Multiple hypermetabolic uptake (maximum SUV: 2.4-4.3) was observed in the right breast axilla, but no other abnormal uptake was seen on whole body PET scans (Fig. 1I).
Subsequent bone marrow biopsy specimen showed diffuse, interstitial infiltration of the marrow space by a monotonous population of blastoid immature cells, and the patient was diagnosed finally as having PTLD of the right breast with comorbid AML relapse, 39 months after allo-PBSCT.
In spite of the 6 months of chemotherapy after diagnosis, the patient died of disseminated fungal infection and multi-organ failure.
Post-transplantation lymphoproliferative disorder is a fatal complication related to solid organ or hematopoetic cell transplantation (3-8). Most PTLDs are B-cell in origin and EBV associated diseases, and T cell lineages are seldom reported, constituting 10-15% of all PTLDs. About 75% of T-cell PTLDs have been reported to be negative for EBV and to behave more aggressively (5, 8-12). In addition, non-EBV-associated PTLDs generally occur later in the post-transplant period compared to the EBV associated PTLDs, and are associated with a poor clinical outcome (3, 5, 8, 9).
This case represents an extremely rare, very late onset, EBV-negative PTLD with monomorphic, immunophenotypical and genotypical characteristics of peripheral T-cell lymphoma NOS, peculiarly involving the breast. So far, only a few cases of T-cell lymphoma or B-cell PTLD of the breast have been reported in literature (11, 13-15). Also, only a few reports represent the imaging features of lymphomatous involvement of the breast (13-15). Mammographic findings in those reports were non-specific and variable; from no detectable abnormal findings to diffusely increased opacities with skin thickening and enlarged axillary lymph nodes, ill-defined or irregular masses to well-defined lesions with a benign appearance (11, 13-15). US features were mostly of solitary, irregular mass to multiple, indistinctly-marginated, mixed hyper- and hypoechoic masses, or pseudocystic configurations. In most cases, 18F-FDG PET-CT showed focal or diffuse hypermetabolism in the breast (11, 13-15). Regardless of the histologic subtype, our case showed similar US features to the reported cases of breast lymphoma; multiple, ill-defined, mixed hyper-and hypo masses, and focal heterogeneous bulging parenchymal lesions. In addition to US, mammography and PET-CT scans brought about confidence in diagnosis of PTLD of the breast. Although imaging findings of PTLD of the breast were not specific, it was not difficult to come up with this rare, but precise diagnosis that considered the imaging features and patient's past medial history.
When PTLD is detected early and treated with reduction of immunosuppressive agents, some can resolve completely. However, in our case, the patient had been diagnosed as having PTLD of the breast and comorbid AML relapse, of which each disease requires a conflicting treatment, finally leading to death of the patient in spite of chemotherapy.
Our case shows that isolated PTLD that involves the breast can be detected by breast US, and with subsequent US-guided core biopsy, a successful pathologic diagnosis can be achieved. In addition, owing to the non-specific US features of PTLD of the breast, further assessments such as mammography or 18F-FDG PET-CT scans may be of assistance towards providing confidence in the diagnosis of this rare form of disease. Although isolated PTLD involving the breast is extremely rare, if a patient with a past medical history of immunosuppressive therapy has palpable symptoms or suspicious abnormality on clinical evaluation involving the breast, imaging studies of the breast should always be considered. Moreover, PTLD should be included in the differential diagnosis of suspicious breast lesions detected in solid organ or hematopoietic cell recipients.
Figures and Tables
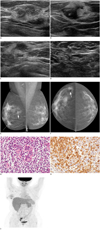 | Fig. 1Twenty two-year-old woman who had undergone allo-PBSCT presenting with vaguely palpable right breast mass.
A-D. Ultrasound shows focal heterogeneous bulging parenchyma area, 2 suspicious irregular hypoechoic masses surrounding echogenic boundaries and right axillary lymph nodes that show nonspecific features with oval shape. E-F. Mammography shows 3, irregular, with ill-defined margin, iso- to slightly hyper-dense masses in right breast and several enlarged lymph nodes which are round to oval shape, some with loss of fatty hilum at right axilla. G. Histologic images of biopsied breast showed diffuse stromal infiltration of mononorphic lymphoid cells without organoid pattern. On high magnification, infiltrated lymphoid cells were small sized, and showed mild nuclear atypism (haematoxylin and eosin staining, × 400). H. Most lymphoid cells are positive for CD3 marker (immunohistochemistry staining, × 400), and negative for EBV (not shown). Overall findings are compatible with monomorphic post-transplant lymphoproliferative disorder, peripheral T cell lymphoma. I. FDG PET shows multiple increased uptake lesions in right breast and hot uptake in right axillary lymph nodes. However, other abnormal uptake is not noted.
|
References
1. Abhyankar SH, Chiang KY, McGuirk JP, Pati AR, Godder KT, Welsh JA, et al. Late onset Epstein-Barr virus-associated lymphoproliferative disease after allogeneic bone marrow transplant presenting as breast masses. Bone Marrow Transplant. 1998; 21:295–297.
2. Au WY, Lie AK, Lee CK, Ma SK, Wan TS, Shek TW, et al. Late onset post-transplantation lymphoproliferative disease of recipient origin following cytogenetic relapse and occult autologous haematopoietic regeneration after allogeneic bone marrow transplantation for acute myeloid leukaemia. Bone Marrow Transplant. 2001; 28:417–419.
3. Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003; 21:1352–1358.
4. Coy DL, Ormazabal A, Godwin JD, Lalani T. Imaging evaluation of pulmonary and abdominal complications following hematopoietic stem cell transplantation. Radiographics. 2005; 25:305–317. discussion 318.
5. Ibrahim HA, Menasce LP, Pomplun S, Burke M, Bower M, Naresh KN. Presence of monoclonal T-cell populations in B-cell post-transplant lymphoproliferative disorders. Mod Pathol. 2011; 24:232–240.
6. Leblond V, Dhedin N, Mamzer Bruneel MF, Choquet S, Hermine O, Porcher R, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001; 19:772–778.
7. Socié G, Curtis RE, Deeg HJ, Sobocinski KA, Filipovich AH, Travis LB, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000; 18:348–357.
8. Yin CC, Medeiros LJ, Abruzzo LV, Jones D, Farhood AI, Thomazy VA. EBV-associated B- and T-cell posttransplant lymphoproliferative disorders following primary EBV infection in a kidney transplant recipient. Am J Clin Pathol. 2005; 123:222–228.
9. Awaya N, Adachi A, Mori T, Kamata H, Nakahara J, Yokoyama K, et al. Fulminant Epstein-Barr virus (EBV)-associated T-cell lymphoproliferative disorder with hemophagocytosis following autologous peripheral blood stem cell transplantation for relapsed angioimmunoblastic T-cell lymphoma. Leuk Res. 2006; 30:1059–1062.
10. Borhani AA, Hosseinzadeh K, Almusa O, Furlan A, Nalesnik M. Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics. 2009; 29:981–1000. discussion 1000-1002.
11. Schramm N, Pfluger T, Reiser MF, Berger F. Subcutaneous panniculitis-like T-cell lymphoma with breast involvement: functional and morphological imaging findings. Br J Radiol. 2010; 83:e90–e94.
12. von Falck C, Maecker B, Schirg E, Boerner AR, Knapp WH, Klein C, et al. Post transplant lymphoproliferative disease in pediatric solid organ transplant patients: a possible role for [18F]-FDG-PET(/CT) in initial staging and therapy monitoring. Eur J Radiol. 2007; 63:427–435.
13. Kiresi DA, Kivrak AS, Ecirli S, Toy H. Secondary breast, pancreatic, and renal involvement with non-Hodgkin's lymphoma: imaging findings. Breast. 2006; 15:106–110.
14. Liberman L, Giess CS, Dershaw DD, Louie DC, Deutch BM. Non-Hodgkin lymphoma of the breast: imaging characteristics and correlation with histopathologic findings. Radiology. 1994; 192:157–160.
15. Yang WT, Lane DL, Le-Petross HT, Abruzzo LV, Macapinlac HA. Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology. 2007; 245:692–702.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download