Abstract
Mediastinal inflammatory pseudotumor is a rare benign disease with its capability for local invasion and rapid growth. We present a case of middle-mediastinal inflammatory pseudotumor and report its contrast-enhanced chest computed tomography, 18F-fluorodeoxyglucose positron emission tomography/computed tomography and pathologic findings.
Inflammatory pseudotumor is an uncommon benign disease mimicking malignancy. It has been reported to occur mostly in young people and to be found in nearly every site of the body, most commonly involving the lungs and orbit (1). The radiographic features are quite nonspecific and various, and the definitive diagnosis is based on the histological evaluation of tissue specimens composed of fibrosis, necrosis, granulomatous reaction and various cell types including histiocytes, myofibroblasts, plasma cells, and lymphocytes (1).
Inflammatory pseudotumor arising purely in the mediastinum is very rare, with only few reported cases in the literature (2, 3). Furthermore, there is only one case presenting the disease with 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) (2). We report a case of middle-mediastinal inflammatory pseudotumor of a patient with a history of acute lymphoblastic leukemia, which showed moderate and heterogeneous 18F-FDG accumulation on PET/CT.
A 25-year-old male had a chest X-ray taken as part of a routine health examination; the results showed a large mass in the right paratracheal space (Fig. 1A). He had had acute lymphoblastic leukemia 11 years earlier but was in complete remission. He was asymptomatic, had no pain, cough, or dyspnea. His physical examination was normal, and all laboratory tests were within normal limits except mild leukocytosis (12.1 × 103/µL, normal range 3.9-9.7). Contrast-enhanced chest CT and 18F-FDG PET/CT were performed to rule out malignant lymphadenopathy (Fig. 1B-D). The mass showed mild homogeneous enhancement with a longitudinal dimension of approximately 8.0 cm in the right paratracheal space in the transaxial view on contrast-enhanced chest CT (Brilliance CT 16-slice, Philips Medical Systems, Cleveland, OH, USA). No abnormality was observed in the lung parenchyma (Fig. 1B). The patient underwent 18F-FDG PET/CT (Discovery STe scanner, GE Healthcare, Milwaukee, WI, USA) after fasting for at least 6 hours. Unenhanced CT was performed by a continuous spiral technique using a 16-slice helical CT (120 KeV, 80-200 mAs with AutomA mode, a section width of 3.75 mm) 60 minutes after injection of 370 MBq 18F-FDG. After CT scan, an emission scan was obtained from the thigh to the head for 3 minutes per frame in the 3-dimensional (3D) mode. Attenuation-corrected PET images using CT data were reconstructed by a 3D ordered-subsets expectation maximization algorithm (20 subsets, 2 iterations). A huge mass with lobulated borders was found in the right paratracheal space; the mean Hounsfield unit of the mass was 36 on unenhanced CT of 18F-FDG PET/CT. On PET images, the mass showed heterogeneous 18F-FDG uptake, with the maximum standardized uptake value (SUVmax) of 5.0 (Fig. 1C, D). No 18F-FDG uptake suggesting metastases was observed on PET/CT.
These clinical and radiological findings suggested malignant lymphadenopathy associated with recurrent leukemia. The patient underwent mediastinoscopic biopsy. Histopathology suggested an inflammatory pseudotumor. The mass was removed via thoracotomy. The tumor was a multinodular mass, measuring about 8 × 6 × 5 cm. On the cut section, the mass was lobulated by intervening fibrous tissue, showing a yellow-brown or yellow-white color with a myxoid character. Histopathologically, the tumor contained multifocal patchy infiltration of B and T lymphoid cells, plasma cells, histiocytes, and fibroblastic cells in a fibrocollagenous stroma (Fig. 1E, F). The final diagnosis was an inflammatory pseudotumor, fibrohistiocytic type.
Inflammatory pseudotumor is an uncommon benign disease of unknown etiology, mimicking malignancy both clinically and radiologically. Trauma, surgical inflammation, immune-autoimmune condition, infection or other malignancies were supposed to be associated with inflammatory pseudotumor (1, 4). Histologically, inflammatory pseudotumor is composed of fibrosis, necrosis, granulomatous reaction and various inflammatory cells including histiocytes, myofibroblasts, plasma cells, and lymphocytes (1). This histologic complexity have caused inflammatory pseudotumor to be described by various names, such as plasma cell granuloma, inflammatory myofibroblastic tumor, histiocytoma, xanthoma, fibroxanthoma, xanthogranuloma, and plasma cell-histiocytoma complex (4). They are most common in the lungs and orbit, but have been reported in nearly every part of the body. They occur most frequently in children and adolescents, but can occur in older persons (1). Patients present with diverse symptoms depending on the site of the lesion, including fever, weight loss, malaise, growth retardation, and symptoms related to mass effect (1). Radiographic findings are nonspecific and various, possibly due to varying degrees of fibrosis, cellular infiltration and dynamic change during the inflammatory process (5). Inflammatory pseudotumor has low, equal, or high attenuation compared with the surrounding tissue on CT. Contrast-enhanced CT may show a homogeneous or heterogeneous lesion. The characteristics of the imaging findings depend on the site of origin of the lesion (1).
Several cases of inflammatory pseudotumor in the lung, liver, kidney, colon and spleen have been reported with 18F-FDG PET/CT images. They showed diverse features, i.e., moderate to intense, and focal to heterogeneous 18F-FDG uptake (1). 18F-FDG PET/CT is highly sensitive, but not specific for the primary diagnosis of inflammatory pseudotumor. However, it could be useful for monitoring treatment response in patients with non-surgical treatment (2, 6).
An inflammatory pseudotumor arising solely in the mediastinum is very rare, and few cases have been reported (2, 3). Among the reported, only one case included 18F-FDG PET/CT images (2). In the report, a subcarinal mass with well-defined borders showed similar attenuation to mediastinal great vessels on unenhanced CT. The PET image demonstrated intense and homogeneous 18F-FDG uptake, but SUVmax was not measured. In our case, 18F-FDG PET/CT demonstrated a huge right paratracheal mass with a similar attenuation value as that of the aortic arch. The 18F-FDG uptake of the mass was moderate and heterogeneous. The 18F-FDG uptake of inflammatory pseudotumor may be high, but its activity and uptake pattern can be varied depending on the amount of fibrosis and cellular components within the mass.
In the present case, the contrast-enhanced chest CT revealed mild homogeneous enhancement in the mass. This feature may reflect mildly increased vascularity of the mass, possibly due to inflammation. However, 18F-FDG uptake was moderate and heterogeneous on the PET images. Increased 18F-FDG uptake may reflect the activity of inflammatory cells. Multifocal and patchy infiltration of inflammatory cells in the lobulated mass by intervening fibrous tissue may result in heterogeneous 18F-FDG uptake of the mass. Such varying degrees and distribution of inflammatory activity may be the reason for the diverse features of 18F-FDG accumulation of inflammatory pseudotumor.
Since the patient had a history of complete remission of acute lymphoblastic leukemia, malignant lymphadenopathy associated with recurrent leukemia was suggested. However, some malignancies such as small cell lung carcinoma, lymphoma and primary malignant fibrous histiocytoma can be presented with a hypermetabolic solitary middle-mediastinal mass on 18F-FDG PET/CT. Thymoma, schwannoma, teratoma, hibernoma, hemangioma and sarcoidosis developing in the middle mediastinum can show 18F-FDG accumulation and are benign (7-10). However, non-FDG-avid tumors such as bronchogenic cysts and pericardial cysts can be excluded from the differential diagnosis of middle-mediastinal inflammatory pseudotumor (10). Therefore, it may be challenging to differentiate an inflammatory pseudotumor from other middle-mediastinal neoplasms based on the pattern of 18F-FDG uptake.
Figures and Tables
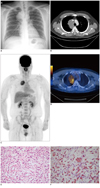 | Fig. 1Imaging and histopathologic findings of mediastinal inflammatory pseudotumor.
A. Chest X-ray showed large mass in right upper mediastinum. B. Contrast-enhanced chest CT demonstrated bulky mass in right paratracheal space with mild homogeneous enhancement. C, D. 18F-FDG PET/CT (C. maximum intensity projection, D. fusion transaxial image) showed heterogeneous FDG accumulation in huge right paratracheal mass (SUVmax = 5.0). SUVmax = maximum standardized uptake value, 18F-FDG = 18F-fluorodeoxyglucose. On histopathologic examination, tumor was composed of lymphoid cells, plasma cells, histiocytes and fibroblastic cells in fibrocollagenous stroma (E. hematoxylin-eosin stain, × 400). Immunohistochemical analysis revealed that CD163 was positive to histiocytes but negative to fibroblastic spindle cells (F. × 400).
|
References
1. Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012; 198:W217–W227.
2. Alongi F, Bolognesi A, Gajate AM, Motta M, Landoni C, Berardi G, et al. Inflammatory pseudotumor of mediastinum treated with tomotherapy and monitored with FDG-PET/CT: case report and literature review. Tumori. 2010; 96:322–326.
3. Chen CH, Lin RL, Liu HC, Chen CH, Hung TT, Huang WC. Inflammatory myofibroblastic tumor mimicking anterior mediastinal malignancy. Ann Thorac Surg. 2008; 86:1362–1364.
4. Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003; 23:719–729.
5. Yoon KH, Ha HK, Lee JS, Suh JH, Kim MH, Kim PN, et al. Inflammatory pseudotumor of the liver in patients with recurrent pyogenic cholangitis: CT-histopathologic correlation. Radiology. 1999; 211:373–379.
6. Obrzut SL, Halpern BS, Monchamp T, Grabski K, Watts WJ, Czernin J. The role of 2-deoxy-2-[(18)F]fluoro-D-glucose positron emission tomography/computed tomography in monitoring the immunosuppressive therapy response of inflammatory myofibroblastic tumor. Mol Imaging Biol. 2004; 6:126–130.
7. Choi BH, Yoon SH, Lee S, Jo KS, Song HS, An YS, et al. Primary malignant fibrous histiocytoma in mediastinum: imaging with 18F-FDG PET/CT. Nucl Med Mol Imaging. 2012; 46:304–307.
8. Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, et al. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. 2006; 7:57–69.
9. Burdick MJ, Jolles PR, Grimes MM, Henry DA. Mediastinal hibernoma simulates a malignant lesion on dual time point FDG imaging. Lung Cancer. 2008; 59:391–394.
10. Kaira K, Abe M, Nakagawa K, Ohde Y, Okumura T, Takahashi T, et al. 18F-FDG uptake on PET in primary mediastinal non-thymic neoplasm: a clinicopathological study. Eur J Radiol. 2012; 81:2423–2429.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download