Abstract
Objective
To compare the accuracy of computed tomography (CT) with that of gastroscopy for the extent of evaluation of longitudinal tumor and type-specific diagnosis of Borrmann type IV gastric cancer.
Materials and Methods
Fifty-nine patients (35 men with mean age of 60 years and 24 women with mean age of 55 years) who underwent surgical resection of Borrmann type IV gastric cancer were included in this study. Histopathological analysis data was used as a reference standard to confirm the clinical interpretations of gastroscopy and CT for the diagnosis of Borrmann type IV and evaluation of longitudinal tumor extent. For the evaluation of longitudinal extent, gastroscopic and CT results were classified as underestimated, accurate, or overestimated. The McNemar test was used to identify statistically significant differences in the accuracy between gastroscopy and CT.
Results
For the diagnosis of Borrmann type IV gastric cancer, the accuracy of CT was significantly higher than that of gastroscopy (74.6% [44/59] vs. 44.1% [26/59], p < 0.001). CT was significantly more accurate in assessing the overall tumor extent than gastroscopy (61.4% [35/57] vs. 28.1% [16/57], p < 0.001). The proximal (75.4% [43/57] vs. 50.9% [29/57], p = 0.003) and distal tumor extent (71.9% [41/57] vs. 43.9% [25/57], p < 0.05) were more accurately predicted by CT compared with gastroscopy. The underestimation of tumor extent was a major source of error in both examinations.
Borrmann type IV gastric cancer, known as scirrhous gastric carcinoma or linitis plastica, shows more frequent involvement of the resection margin following surgical resection and worse prognosis than those with other Borrmann types of advanced gastric cancer (AGC) (1, 2). The diagnosis and determination of the exact tumor extent of type IV AGC due to its infiltrative growth pattern (1-3). Since, it has been reported that neoadjunvant chemotherapy could be a promising treatment option in patients with Borrmann type IV gastric cancer (4), accurate preoperative diagnosis and determination of the longitudinal extent of Borrmann type IV AGC are vital in predicting the prognosis and in designing optimal therapeutic strategy (5).
Computed tomography (CT) coupled with gastroscopy is a mainstay in the preoperative diagnosis and staging of gastric cancer (6-10). Gastroscopy has also been used in the preoperative diagnosis, localization and determination of longitudinal tumor extent in patients with gastric cancer (10, 11). Since gastroscopy mainly focused on the mucosal side of the stomach, its used is limited in the diagnosis and assessment of the tumor extent (3, 12, 13), such as Borrmann type IV gastric cancers have a unique infiltrative growth pattern along the submucosal layer without prominent mucosal mass or ulcer (1, 2, 14-17).
Literature detailed the imaging features of Borrmann type IV AGC on upper gastrointestinal (UGI) series (12, 13) and CT (12, 13, 18, 19). However, to best our knowledge, no study has been conducted to address the value of CT in type-specific diagnosis of Borrmann type IV cancer and evaluating the longitudinal tumor extent.
The purpose of the present study was to retrospectively compare the accuracy of CT with that of gastroscopy in the evaluation of longitudinal tumor extent as well as the type-specific diagnosis of Borrmann type IV gastric cancer.
Institutional review board retrospective approved the study and patient informed consent was waived. A computerized search of medical records identified 1228 patients who underwent surgery for gastric cancer at our institution between May 2003 and July 2007. Borrmann type IV AGC was defined as diffusely infiltrating carcinomas in which ulceration is usually not a marked feature based on the surgical and pathological findingst (20), Borrmann type IV AGC was confirmed in 72 consecutive patients after gastric surgery. Of these, 13 patients were excluded for the reasons such as patients who did not undergo gastroscopy (n = 4) or CT (n = 1) at our institution, only palliative gastrojejunostomy without gastrectomy was performed due to the presence of distant metastasis (n = 4), patients underwent preoperative neoadjuvant chemotherapy for gastric cancer (n = 2) and previous history of partial gastrectomy (n = 1), synchronous gastric cancer (n = 1). Finally, 59 patients (mean age, 58 years; range, 30-79 years) who underwent preoperative gastroscopy and CT followed by gastrectomy at our institution were included in our study.
The study population included 35 men (mean age, 60 years; range, 30-79 years) and 24 women (mean age, 55 years; range, 30-74 years) and the mean time interval between gastroscopy and surgery was 13.2 days (range, 1-76 days) and between CT and surgery was 10.1 days (range, 1-40 days). Of the 36 patients in whom gastroscopy was performed prior to CT examination (mean interval, 7.2 days; range, 0-74 days), seven patients underwent gastroscopy as a preliminary evaluation for the abdominal symptoms and 29 patients underwent a second gastroscopic examination to the detection of gastric lesions in the preliminary examination. Prior to gastroscopic procedure, endoscopists were made aware of the mecal history and clinical findings of each patient including the reason for undergoing gastroscopy and preliminary findings. Radiologists, who analyzed each CT examinations after gastroscopy, were made available the gastroscopic examination data including type, location and extent of the gastric lesion.
Twenty-three patients underwent CT examination prior to gastroscopy (mean interval, 4.1 days; range, 0-16 days), with two patients underwent multidetector row computed tomography examinations as an initial diagnostic modality for abdominal symptoms of the patients without any suspicion of gastric cancer and in the 21 patients, although radiologists were aware of the suspicion for gastric cancer, they did not know about type, location, and extent of the gastric lesion. During the examination session for each gastroscopy, CT results including the characteristics of the gastric lesion were fully available for the endoscopists (Fig. 1).
Gastroscopy was performed by one of three endoscopists (with 6, 16, and 17 years of experience, respectively) using various type of endoscopic unit (GIF Q-260, XQ-260 or XP-260; Olympus Optical Co., Ltd., Tokyo, Japan). Prior to the examinations, the endoscopists reviewed the medical record, including the CT report, if available, and aware of the history and clinical findings of the patient including the reason for undergoing gastroscopy and initial gastroscopic result. For gastroscopy, the presence and extent of type IV AGC were determined according to enlarged or effaced rugal folds, circumferentially infiltrating lesions or loss of distensibility despite air insufflation associated with hyperemic mucosal change and small erosions (12, 13, 21). The diagnosis and longitudinal extent of the tumor were recorded according to the Japanese classification of gastric cancer (JCGC) (20). The proximal tumor extent was determined as one of the distal esophagus, upper third of the stomach, middle third of the stomach and lower third of the stomach. The distal tumor extent was determined as one of the upper third of the stomach, middle third of the stomach, lower third of the stomach and the proximal duodenum.
Contrast-enhanced CT examinations were performed using 16- (n = 51) or 64- (n = 8) detector-row scanners (Brilliance; Philips Medical Systems, Cleveland, OH, USA) in all patients. Each patient was asked to drink 500-1000 mL of tap water for gastric distension before the CT examination. Intravenous nonionic contrast material (2 mL/kg; iopromide, Ultravist 370; Schering, Berlin, Germany) was administered via the antecubital vein, using a power injector (Stellant D, Medrad, Indianola, PA) at a rate of 3 mL/sec. Bolus-tracking software (Brilliance; Philips Medical Systems, Cleveland, OH, USA) was used to trigger the scanning 60 seconds after the aortic enhancement reached a 150-HU threshold. Helical scan data were acquired using 16 × 1.5 mm or 64 × 0.625 mm collimation, a rotation speed of 0.5 or 0.42 sec, a pitch of 1.11 to 1.25, and 120 kVp. Effective mAs ranged from 118 to 195 mAs using an automatic tube current modulation technique (Dose-Right; Philips Medical Systems, Cleveland, OH, USA). CT scan was performed in supine (n = 4) or prone (n = 55) position. Transverse and coronal section datasets were reconstructed with a section thickness of 4-mm thick at 3-mm increments.
Two abdominal radiologists (with ten- and nine-year experience of oncologic imaging, respectively) reviewed the CT images as a part of daily routine practice. Radiologists completed a structured report that included Borrmann type, location and extent of tumor. The two radiologists had. Each radiologist interpreted 31 and 28 studies, respectively. Before the interpretation, the radiologists reviewed the medical records and aware of patients clinical findings including the results of gastroscopic examination including type, location and extent of the gastric lesion if available. The diagnosis and longitudinal tumor extent of Borrmann type IV AGC were determined in a manner same to that of gastroscopy. The findings indicating Borrmann type IV gastric cancer included diffuse or segmental thickening with prominent enhancement of the gastric wall, marked hypertrophy or obliteration of gastric mucosal folds or concentric stricture (12, 13, 18, 19, 22). The proximal and distal tumor margins on CT were determined as the proximal and distal margins of enhancing thickening of the gastric wall.
Surgical exploration was carried out, to determine the extent of gastric resection, by one or two surgeons (with 15 and 6 years of gastric surgery experience, respectively) based on the tumor location and extent as suggested by gross inspection and palpation as well as the results of gastroscopy and CT examinations. Immediately after subtotal or total gastric resection, frozen biopsy of the resection margin was routinely performed and the analyses of frozen biopsy was carried out to determine the need for the further resection. Histopathological examination of the resected specimen in 59 patients, by a pathologist confirmed the Borrmann type and longitudinal tumor extent as well as TNM according to the published guidelines (20). Tumor involvement at the proximal and distal resection margins was also determined.
A study coordinator reviewed the reports of preoperative gastroscopy and CT for diagnosis of Borrmann type IV cancer and longitudinal tumor extent. The results from the gastroscopy and CT reports were compared with those from the histopathological report as a reference standard. If the Borrmann type recorded in the gastroscopy or CT reports was type IV, the gastroscopy or CT interpretations were considered to be accurate. For the evaluation of longitudinal tumor extent, the gastroscopy and CT results were classified as underestimated, accurate, or overestimated compared to the reference standard separately for the proximal and the distal tumor margins. An underestimation of the proximal and distal tumor extent was assumed, when the gastroscopy- or CT-estimated tumor margins were distal and proximal, respectively, to the pathologically confirmed tumor extent. An accurate evaluation was considered when the gastroscopy or CT evaluation matched the pathologically confirmed tumor extent. An overestimation of the proximal and distal tumor extent was predicted when the gastroscopy- or CT-evaluated margins were proximal and distal, respectively, to the pathologically confirmed tumor extent. An precise evaluation of the overall tumor extent was defined as when the gastroscopy or CT evaluation coincided with the pathologically confirmed tumor extent for both the proximal and the distal margins. The McNemar test was used to identify statistically significant differences in the accuracy between gastroscopy and CT. P-values of less than 0.05 was considered statistically significant. Statistical software (MedCalc, version 9.2.0.1; MedCalc Software, Mariakerke, Belgium) was used for statistical analyses.
Thirty-eight and 17 patients underwent total and subtotal gastrectomy, respectively, in these four patients, conversion of initial subtotal gastrectomy to total gastrectomy was performed during the surgery due to the involvement of residual tumor at the resection margin. Combined partial resection of the esophagus or duodenum was performed in 25 patients (esophagus, n = 9; duodenum, n = 11; and both, n = 5). The confirmed longitudinal tumor extents are summarized in Table 1. In nine (15.3%) of the 59 patients, residual tumor was present at the resection margin on the histopathologic examination. Two and six patients had residual tumor in the proximal and distal margin, respectively. One patient had residual tumor involvement at both proximal and distal margins. All of the positive proximal margins and positive distal margins were located at the esophagus and duodenum, respectively. In these nine patients, although the presence of residual tumor cells had already been noted on frozen biopsy of resection margin during operation, the surgeon could not perform further resection of the esophagus or duodenum due to concerns of anastomotic failure. Thus, these nine patients underwent gastrectomy with microscopic residual tumor (R1 resection). The T stage was T2 in ten patients (16.9%), T3 in 43 patients (72.9%) and T4 in six patients (10.2%). The N stage was N0 in seven patients (11.9%), N1 in 14 patients (23.7%), N2 in 10 patients (16.9%), and N3 in 28 patients (47.5%) as classified in the 2nd edition of JCGC guidelines (20).
Table 2 presents the results of preoperative diagnosis of Borrmann type IV gastric cancer on gastroscopy and CT. On gastroscopy, Borrmann type IV AGC were correctly diagnosed in 26 (44.1%, 26/59) patients (Table 2). In 15 patients (57.6%, 15/26), the presence of gastric cancer had been suspected on gastroscopy performed at referring hospitals. Thirty-one patients were diagnosed as other Borrmann types of AGC or early gastric cancer (Fig. 2). Two (3.4%) patients were diagnosed as benign pyloric stenosis due to recurrent duodenal ulcer (Fig. 3). On preoperative CT, Borrmann type IV AGC was correctly diagnosed in 44 (74.6%, 44/59) patients (Figs. 2, 3) (Table 2). Of these 44 patients, 25 (56.8%, 25/44) patients were suspected of having gastric cancer by gastroscopy prior to CT examination. For the type-specific diagnosis of Borrmann type IV AGC, the accuracy of the CT examination was significantly higher than that of gastroscopy (p < 0.001).
For the analysis of the evaluation of longitudinal tumor extent, two patients were excluded due the presence of benign pyloric stenosis at gastroscopy (Fig. 3). Thus, longitudinal tumor extent analysis was performed in 57 patients.
At gastroscopy, the proximal tumor extent was underestimated, accurately estimated and overestimated in 24 (42.1%), 29 (50.9%) and 4 (7.0%) of the 57 patients, respectively. In CT, the proximal tumor extent was underestimated, accurately evaluated, and overestimated in 11 (19.3%), 43 (75.4%) and 3 (5.3%) of the 57 patients, respectively (Table 3). CT was significantly more accurate than gastroscopy in evaluating the proximal tumor extent (p = 0.003). In 22 patients who initially underwent CT prior to gastroscopy, CT demonstrated significantly higher accuracy in determining the proximal tumor extent than gastroscopy (77.3% [17/22] vs. 45.5% [10/22], p = 0.015).
Among the four categories of the proximal tumor extent, CT showed particularly more accurate evaluation of esophageal invasion than gastroscopy (92.9% [13/14] vs. 50% [7/14], p = 0.03). Eleven of fourteen patients who were confirmed to have an esophageal invasion on the pathologic examination could undergo curative surgery.
In the 57 patients, gastroscopy underestimated, accurately evaluated, and overestimated the distal tumor extent in 29 (50.9%), 25 (43.9%) and three (5.3%) patients, respectively. At CT, the distal tumor extent was underestimated, accurately evaluated and overestimated in 13 (22.8%), 41 (71.9%) and 3 (5.3%) of the 57 patients, respectively (Table 4). CT was significantly more accurate than gastroscopy in evaluating the distal tumor extent (p < 0.05). Also, CT showed significantly higher accuracy in evaluating the distal tumor extent than gastroscopy in 22 patients who initially underwent CT before gastroscopy (68.2% [15/22] vs. 40.9% [9/22], p = 0.03).
Among the four categories of distal tumor extent, CT demonstrated significantly higher accuracy in evaluating duodenal invasion in particular, than gastroscopy (60% [9/15] vs. 7% [1/15], p = 0.007) (Fig. 4). Eight of fifteen patients who were proved to have duodenal invasion on the pathologic examination could undergo curative surgery.
The overall tumor extent was accurately evaluated in 16 (28.1%) and 35 (61.4%) of the 57 patients with gastroscopy and CT, respectively (p < 0.001). In 22 patients who initially underwent CT before gastroscopy, CT was significantly more accurate than gastroscopy in evaluating total tumor (54.5% [12/22] vs. 22.7% [5/22], p = 0.01).
In the present study, the accuracy of CT (74.6%) for the preoperative type-specific diagnosis of Borrmann type IV AGC was higher than that of gastroscopy (44.1%). Even though gastroscopy combined with biopsy showed a high sensitivity of 90-98% in the detection of gastric cancer (10, 23), in the case of Borrmann type IV AGC, however, not only detection of the tumor but also diagnosis of the exact type of tumor has been documented as being difficult with gastroscopy (12, 13, 21, 24). Furthermore, although gastroscopic biopsy was performed with suspicion of Borrmann type IV AGC, the positive rate for cancer diagnosis on biopsy specimen was also significantly lower in Borrmann type IV AGC than other Borrmann types of AGC (12, 21, 25). These poor performances of gastroscopy and combined biopsy reflect the unique growth and morphologic characteristics of Borrmann type IV cancer. Since these tumors are located mainly in the submucosal layer and there is no prominent ulcer or mass on the mucosal surface, it is difficult for endoscopists to recognize the lesion (1, 12-17). Also, since cancer cells are often widely scattered within a dense fibrous stroma from associated desmoplastic reaction and spare the mucosal layer, it may also be difficult for a pathologist to diagnose cancer from a small biopsy specimen that often contains only the mucosal layer (12, 13, 25). Hence, it is suggested that the diagnosis of Borrmann type IV AGC by CT would be helpful for endoscopists or pathologists to make correct diagnosis. In contrast to gastroscopy, CT can better depict the strongly enhanced mural thickening which indicates tumor infiltration of Borrmann type IV AGC, even in the case of gastric outlet obstruction that can hinder gastroscopic examination (18, 19, 26).
In our results, CT was more accurate than gastroscopy (61.4% vs. 28.1%) for the evaluation of the longitudinal tumor extent. According to previous studies comparing gastroscopy with UGI series (12, 13), gastroscopy showed a limited performance to evaluate type-specific diagnosis and tumor extent in patients with Borrmann type IV AGC. According to a study by Kitamura et al. (14), the tumor location and extent were diagnosed correctly only in 24 of 72 patients (33%) on gastroscopy, which is comparable to our results here. The limitation of gastroscopy in patients with type IV AGC is also likely due to the infiltrative tumor growth along submucosal layer that is often associated with the normal-appearing mucosal surface (1, 12-17). In particular, the sensitivity of endoscopy in the determination of esophageal and duodenal invasion of scirrhous gastric cancer is poor. It could be due to the fact that the invasion of gastric cancer to the esophagus and duodenum is often infiltrative, directly through the submucosal or subserosal layer. Other main reasons for the poor sensitivity of gastroscopy are that both of the gastroesophageal junction and pyloric ring have a tubular shape with a narrow lumen, making it difficult to observe the tumor infiltration on endoscopy and the strong peristalsis of the esophagus and duodenum could further hinder the endoscopist from making a satisfactory observation (27-29). On the contrary, CT can better depict the tumor infiltration along the stomach and into esophagus and duodenum with prominent enhancement (12, 13, 18, 19, 22). In our study, CT demonstrated significantly higher accuracy in evaluating esophageal and duodenal invasion than gastroscopy.
Underestimation of the tumor extent occurred more frequently (19.3% in proximal extent, 22.8% in distal extent) than overestimation (5.3% in proximal and distal extent) by CT, however, CT was reportedly more accurate than gastroscopy for the evaluation of the longitudinal tumor extent. In partivular, the underestimation of duodenal invasion was more significant compared to the analysis of esophageal invasion in CT and gastroscopy. Although the exact reason why the underestimation of duodenal invasion was higher than the underestimation of esophageal invasion cannot be simply be explained, the tumor invasion into distal esophagus that is scanned tangentially and visualized as the target-like structure in the axial image would be more easily recognized than invasion into duodenum that is scanned with oblique plane. Underestimation of the tumor extent can result in either unexpected wider resection of the gastrointestinal tract or tumor involvement at the gastrectomy margins. In cases of underestimation of proximal margin, unexpected conversion of subtotal gastrectomy to total gastrectomy should be undergone during the operation. In the present study, four patients had to undergo conversion of initial subtotal gastrectomy to total gastrectomy during the operation owing to involvement of residual tumor at the resection margin on frozen biopsy. Even on the final histopathologic examination for the resected specimen, residual tumor was detected at the surgical margin of nine (15.3%, 9/59) patients. Our results are comparable with previous studies reporting frequent tumor involvement of the resection margins in Borrmann type IV AGC than in other Borrmann types of AGC (2, 14, 30, 31). Since the underestimation for tumor extent and residual tumor on resection margin in type IV AGC is frequent, endoscopists and radiologists need to pay particular attention to determine the longitudinal tumor extent, especially the distal extent and keep in mind the possibility of tumor infiltration beyond the estimated extent on both gastroscopy and CT. In two patients, the complete examination of the primary lesion was not possible by gastroscopy due to gastric outlet obstruction, and diagnosed as benign pyloric stenosis due to recurrent duodenal ulcer and not suspected to have a gastric cancer on gastroscopy. Thus, the endoscopists could not assess the extent of tumor. However, radiologists could correctly diagnose the type IV AGC involving the lower third of the stomach and duodenum and accurately determined the proximal and distal tumor extents at CT. Therefore, in the case of pyloric stenosis, CT may be helpful to detect tumor involvement which can be inaccessible on gastroscopy.
The limitations of the present study include the retrospective nature of the analyses and clinical interpretation results from two radiologists, rather than retrospectively reviewing the CT examinations for this study. Although the retrospective review for the CT images with being blind to the gastroscopic finding may avoid the reader bias, however, we believe that our results from the routine clinical practice may better reflect the performance of gastroscopy and CT in practice. Secondly, we only included the patients who underwent surgical resection of the primary tumor; therefore, there could be a bias towards less-advanced cases. Finally, 36 patients underwent gastroscopic examination prior to CT examination at our institution. Since, the radiologists were already aware of the results of gastroscopic examinations, this un-blinding analysis can result in the reader bias and results regarding the accuracy of CT could be overestimated. However, we can identify that the CT was more accurate than endoscopy of evaluating tumor extent in 22 patients who underwent CT prior to gastroscopy. Moreover, in routine clinical practice, where CT plays a major role in the preoperative staging of gastric cancer, it is more desirable to utilize all available clinical information including gastroscopic results when interpreting CT, in terms of patient benefits and ethics. Furthermore, this study was planned to reflect the modern real clinical situation of practice, where radiologists could be fully exposed to a patient's history and results from other examinations with electronic medical record. Although radiologists could be affected by previous gastroscopic results, and vice versa, we believe that our results could readily reflect the real performance of CT in practical situation. In addition, the CT images in our study were obtained with single phase scan. Given prominent desmoplastic reaction and infiltrative growth pattern of Borrmann type IV AGC, additional delayed phase scan might be helpful to better characterize the longitudinal extent of this tumor. In addition, in seven patients who underwent preliminary gastroscopy for abdominal symptoms, gastroscopist was primarily concerned not to evaluate the tumor extent but to detect abnormal lesion including cancer. This situation may make a bias to lower the accuracy of gastroscopy for evaluating the tumor extent. Sixth, according to the previous report (32), patient position can affect the gastric distension and lesion conspicuity in CT gastrography using air distension. The left posterior oblique or supine position combined with prone position could achieve the good gastric distension in CT gastrography. In our study, CT scan was performed in prone position after drinking of water for the most of patients (55 of 59 patients). Single scan position might result in the suboptimal gastric distension and subsequently affect the CT interpretation results.
In conclusion, CT is more accurate than gastroscopy in the type-specific diagnosis and evaluation of the longitudinal tumor extent of Borrmann type IV AGC. However, both CT and gastroscopy frequently underestimate the longitudinal tumor extent, which requires a through evaluation.
Figures and Tables
 | Fig. 1
Flow chart of study profile based on recommended standards for reporting diagnostic accuracy. AGC = advanced gastric cancer, MDCT = multidetector row computed tomography |
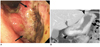 | Fig. 2Fifty four-year-old man with Borrmann type IV gastric carcinoma involving upper, middle, and lower third of stomach.
Preoperative diagnosis was Borrmann type III AGC involving lower third at gastroscopic examination and Borrmann type IV AGC involving upper, middle, and lower third of stomach at CT examination. (A) Gastroscopic image reveals infiltrative mass (arrows) with depressed area which is encircling prepyloric antrum. (B) Coronal CT images show abnormal strong enhancement in upper, middle, and lower third of gastric wall, accompanied by wall thickening and hypertrophied gastric mucosal folds (arrowheads). AGC = advanced gastric cancer, CT = computed tomography
|
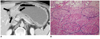 | Fig. 3Forty four-year-old man with Borrmann type IV gastric carcinoma involving lower third of stomach and duodenum.
Preoperative diagnosis was pyloric stenosis due to recurrent duodenal ulcer with bulb deformity at gastroscopic examination and Borrmann type IV AGC involving lower third of stomach and duodenum at CT examination. (A) Axial image demonstrate highly enhanced, concentric wall thickening of pyloric antrum and duodenum with obstruction of gastric outlet (arrows). (B) Photomicrograph shows multifocal and discontinuous infiltrations of tumor cell clusters along gastric wall. Circles indicate regions where tumor cells invade submucosal layer, sparing superficial mucosal layer (Hematoxylin-eosin stain; original magnification, × 100). AGC = advanced gastric cancer, CT = computed tomography
|
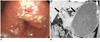 | Fig. 4Fifty five-year-old man with Borrmann type IV gastric carcinoma involving lower third of stomach and proximal duodenum.
Preoperative diagnosis was Borrmann type IV AGC involving lower third of stomach at gastroscopic examination and type IV AGC involving lower to mid third stomach and duodenum. (A) Gastroscopic image shows infiltrative lesion involving prepyloric anturm and pylorus ring without evidence of invasion into duodenum (B) Coronal image shows thickened gastric wall with enhancement from lower to mid third of stomach and involvement of duodenal bulb (arrows). Pathologic examination revealed type IV AGC involving lower to mid third of stomach and duodenal invasion. AGC = advanced gastric cancer, CT = computed tomography
|
Table 2
Preoperative Evaluation of Borrmann type IV Gastric Cancer on Gastroscopy and CT Examination

Table 3
Determination of Proximal Tumor Margin with Gastroscopy and CT in 57 Patients with Borrmann Type IV Gastric Cancer

References
1. Nakamura R, Saikawa Y, Wada N, Yoshida M, Kubota T, Kumai K, et al. Retrospective analysis of prognosis for scirrhous-type gastric cancer: one institution's experience. Int J Clin Oncol. 2007; 12:291–294.
2. Kim DY, Kim HR, Kim YJ, Kim S. Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ J Surg. 2002; 72:739–742.
3. Haruma K, Yoshihara M, Tanaka S, Sumii K, Kajiyama G, Hidaka T, et al. Rapid growth and difficulty of early detection of scirrhous carcinoma of the stomach. Am J Gastroenterol. 1992; 87:31–36.
4. Sun XC, Lin J, Ju AH. Treatment of Borrmann type IV gastric cancer with a neoadjuvant chemotherapy combination of docetaxel, cisplatin and 5-fluorouracil/leucovorin. J Int Med Res. 2011; 39:2096–2102.
5. Nazli O, Derici H, Tansug T, Yaman I, Bozdag AD, Isgüder AS, et al. Survival analysis after surgical treatment of gastric cancer: review of 121 cases. Hepatogastroenterology. 2007; 54:625–629.
6. Chen BB, Liang PC, Liu KL, Hsiao JK, Huang JC, Wong JM, et al. Preoperative diagnosis of gastric tumors by three-dimensional multidetector row ct and double contrast barium meal study: correlation with surgical and histologic results. J Formos Med Assoc. 2007; 106:943–952.
7. Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim PN, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005; 236:879–885.
8. Kumano S, Murakami T, Kim T, Hori M, Iannaccone R, Nakata S, et al. T staging of gastric cancer: role of multi-detector row CT. Radiology. 2005; 237:961–966.
9. Hur J, Park MS, Lee JH, Lim JS, Yu JS, Hong YJ, et al. Diagnostic accuracy of multidetector row computed tomography in T- and N staging of gastric cancer with histopathologic correlation. J Comput Assist Tomogr. 2006; 30:372–377.
10. Hakim NS, Sarr MG, van Heerden JA. Does endoscopy really help the surgeon evaluate gastric cancer? Can J Surg. 1989; 32:175–177.
11. Abdalla EK, Pisters PW. Staging and preoperative evaluation of upper gastrointestinal malignancies. Semin Oncol. 2004; 31:513–529.
12. Levine MS, Kong V, Rubesin SE, Laufer I, Herlinger H. Scirrhous carcinoma of the stomach: radiologic and endoscopic diagnosis. Radiology. 1990; 175:151–154.
13. Park MS, Ha HK, Choi BS, Kim KW, Myung SJ, Kim AY, et al. Scirrhous gastric carcinoma: endoscopy versus upper gastrointestinal radiography. Radiology. 2004; 231:421–426.
14. Kitamura K, Beppu R, Anai H, Ikejiri K, Yakabe S, Sugimachi K, et al. Clinicopathologic study of patients with Borrmann type IV gastric carcinoma. J Surg Oncol. 1995; 58:112–117.
15. Whitehead R, Johansen A. Other tumors of the stomach. In : Whitehead R, editor. Gastrointestinal and oesophageal pathology. 2nd ed. New York, NY: Churchill Livingstone;1995. p. 823–836.
16. Silverstein FE, Tytgat GNJ. Stomach II: tumors and polyps. In : Silverstein FE, Tytgat GNJ, editors. Gastrointestinal endoscopy. 3rd ed. London, England: Mosby-Wolfe;1997. p. 147–180.
17. Yokota T, Teshima S, Saito T, Kikuchi S, Kunii Y, Yamauchi H. Borrmann's type IV gastric cancer: clinicopathologic analysis. Can J Surg. 1999; 42:371–376.
18. Minami M, Kawauchi N, Itai Y, Niki T, Sasaki Y. Gastric tumors: radiologic-pathologic correlation and accuracy of T staging with dynamic CT. Radiology. 1992; 185:173–178.
19. Balthazar EJ, Siegel SE, Megibow AJ, Scholes J, Gordon R. CT in patients with scirrhous carcinoma of the GI tract: imaging findings and value for tumor detection and staging. AJR Am J Roentgenol. 1995; 165:839–845.
20. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998; 1:10–24.
21. Winawer SJ, Posner G, Lightdale CJ, Sherlock P, Melamed M, Fortner JG. Endoscopic diagnosis of advanced gastric cancer. Factors influencing yield. Gastroenterology. 1975; 69:1183–1187.
22. Balthazar EJ, Rosenberg H, Davidian MM. Scirrhous carcinoma of the pyloric channel and distal antrum. AJR Am J Roentgenol. 1980; 134:669–673.
23. Voutilainen ME, Juhola MT. Evaluation of the diagnostic accuracy of gastroscopy to detect gastric tumours: clinicopathological features and prognosis of patients with gastric cancer missed on endoscopy. Eur J Gastroenterol Hepatol. 2005; 17:1345–1349.
24. Evans E, Harris O, Dickey D, Hartley L. Difficulties in the endoscopic diagnosis of gastric and oesophageal cancer. Aust N Z J Surg. 1985; 55:541–544.
25. Kanter MA, Isaacson NH, Knoll SM, Nochomovitz LE. The diagnostic challenge of metastatic linitis plastica. Two cases and a consideration of the problem. Am Surg. 1986; 52:510–513.
26. Ba-Ssalamah A, Prokop M, Uffmann M, Pokieser P, Teleky B, Lechner G. Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics. 2003; 23:625–644.
27. Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003; 362:305–315.
28. Matsumoto M, Azuma M, Shimoda W, Tanaka N, Okawa T, Yamaguchi N, et al. A study of early gastric cancer in the vicinity of the pylorous ring or invading the duodenum. J Jpn Surg Assoc. 2000; 61:22–26.
29. Kakeji Y, Tsujitani S, Baba H, Moriguchi S, Mori M, Maehara Y, et al. Clinicopathologic features and prognostic significance of duodenal invasion in patients with distal gastric carcinoma. Cancer. 1991; 68:380–384.
30. An JY, Kang TH, Choi MG, Noh JH, Sohn TS, Kim S. Borrmann type IV: an independent prognostic factor for survival in gastric cancer. J Gastrointest Surg. 2008; 12:1364–1369.
31. Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T, Ohkubo H. Clinicopathologic characteristics of gastric cancer patients with cancer infiltration at surgical margin at gastrectomy. Anticancer Res. 1997; 17:689–694.
32. Kim HJ, Kim AY, Lee JH, Yook JH, Yu ES, Ha HK. Positioning during CT gastrography in patients with gastric cancer: the effect on gastric distension and lesion conspicuity. Korean J Radiol. 2009; 10:252–259.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download