Abstract
Objective
To determine the reliable perfusion parameters in dynamic contrast-enhanced MRI (DCE-MRI) for the monitoring antiangiogenic treatment in mice.
Materials and Methods
Mice, with U-118 MG tumor, were treated with either saline (n = 3) or antiangiogenic agent (sunitinib, n = 8). Before (day 0) and after (days 2, 8, 15, 25) treatment, DCE examinations using correlations of perfusion parameters (Kep, Kel, and AH from two compartment model; time to peak, initial slope and % enhancement from time-intensity curve analysis) were evaluated.
Results
Tumor growth rate was found to be 129% ± 28 in control group, -33% ± 11 in four mice with sunitinib-treatment (tumor regression) and 47% ± 15 in four with sunitinib-treatment (growth retardation). Kep (r = 0.80) and initial slope (r = 0.84) showed strong positive correlation to the initial tumor volume (p < 0.05). In control mice, tumor regression group and growth retardation group animals, Kep (r : 0.75, 0.78, 0.81, 0.69) and initial slope (r : 0.79, 0.65, 0.67, 0.84) showed significant correlation with tumor volume (p < 0.01). In four mice with tumor re-growth, Kep and initial slope increased 20% or greater at earlier (n = 2) than or same periods (n = 2) to when the tumor started to re-grow with 20% or greater growth rate.
Suppression of angiogenesis isa promising strategy for treatment of cancer, and several antiangiogenic agents have been developed to cancer treatment, which selectively lead to structural and functional degradation of tumor vessels (1).
For monitoring of antiangiogenic treatment, dynamic contrast-enhanced magnetic resonance (DCE-MR) imaging is a useful modality as it can trace the changes of various perfusion-related parameters on repeated examinations after the initiation of antiangiogenic treatment. Several studies have suggested the feasibility of DCE-MR for the monitoring of antiangiogenic treatment with rapid changes of various perfusion parameters post to antiangiogenic treatment (2, 3). However, since as many tumors recur or re-grow despite the early positive responses to treatment, it is debatable that early change of some perfusion parameters can be simply adopted as surrogates for determining the treatment results. Moreover, it is controversial which parameters are accurate and reliable for monitoring with DCE-MR to evaluate the outcomes of effect of antiangiogenic treatment (4, 5), particularly, changes invarious perfusion parameters on the follow-up DCE-MR examinations usually lead to inconclusive endpoints after treatment. Therefore, it is necessary to evaluate each perfusion parameter for their reliability in depicting the changes of angiogenic activity in a longitudinal study.
The purpose of the present study was to determine the optimal perfusion parameters for monitoring of antiangiogenic treatment in DCE-MR in a longitudinal study.
The study protocol was approved by the local ethics committee for animal care and use.
Female BALB/c-nu mice (6-7 weeks old; n = 12, SLC, Shizuoka, Japan) were implanted subcutaneously with 1 × 107 U-118 MG cells (ATCC HTB-15), with strong activity of vascular endothelial growth factor receptor (VEGFR), into the flank region (6). After 14 days of tumor cell implantation (day 0), MR examinations were initiated and the day following the initial MR examination, tumor-bearing mice were received sunitinib malate (Sutent, Pfizer, New York, NY, USA) (treatment group, n = 8, 80 mg/kg/day) or saline (control group, n = 4) daily by oral gavage. The sunitinib is a selective receptor tyrosine kinase inhibitor that exhibits direct antitumor activity against tumor cells dependent on signaling through VEGFR (7). The dose of sunitinib was determined based on an earlier study showing greatest reduction of tumor volume (7).
The animals were examined using a 4.7-T Biospec MR imaging system (Bruker, Karlsruhe, Germany), equipped with a maximum gradient strength of 400 mT/m (bore diameter, 12 cm). Radiofrequency transmission and reception were performed with a quadrature volume coil (diameter, 35 mm). MR examinations were performed prior to (day 0) and after (days 2, 8, 15, 25) administration of sunitinib or saline. The deceased mice were excluded from the study during the follow-up period.
MR imaging was conducted under isoflurane anesthesia (1.0-1.5%) in 69% N2O and 30% O2. A mean body temperature of 37℃ ± 1 was maintained with warm air flow.
We initially obtained axial T2-weighted images to measure tumor volume using rapid acquisition with relaxation enhancement sequence according to the following parameters: TR, 5000 msec; TE, 22.5 msec; slice thickness, 1 mm; no gap; matrix size, 256 × 256; and field of view, 25 × 25 mm2. The follow-up, DCE-MR images were obtained using a dynamic series of gradient echo images with the following parameters: TR, 20 ms; TE, 2.14 ms; flip angle, 30° FOV, 25 × 25 mm2; matrix size, 128 × 128 and number of repetition, 400. The contrast agent (Dotarem, Guerbet, France) was injected as a bolus into the tail vein at a dose of 0.1 mmol/kg at 30 seconds following the start of image acquisition. Total scanning time per tumor was approximately 17 min as the temporal resolution for each scanning was 2.55 seconds.
The volume of viable tumor was measured on T2-weighted images while referring to dynamic contrast-enhanced images as viable tumor was defined as enhancing areas within tumors. A radiologist marked theregions-of-interest (ROIs) including the entire viable tumor areas throughout the images followed by the stacks of ROIs in each tumor were turned into a three-dimensional volume-of-interest. The same ROIs were applied for measuring tumor volume and hemodynamic parameters.
Voxel-based fitting of time-intensity curves was performed according to Brix two-compartment model, as described previously (8, 9)
where S(0) is signal intensity before contrast injection, S(t) is signal intensity at the time t, Kep is the efflux rate constant from extravascular extracellular space to blood plasma, thereby indicating the permeability, Kel is the first-order elimination rate constant of Gd-DTPA from plasma, and AH is the constant representing the size of the extravascualar extracellular space (8). Three additional parameters were estimated from the time-intensity curves (10) namely the time to peak indicating the duration from the onset of contrast enhancement to the peak signal intensity, initial slope, which indicated the differential at the onset of contrast enhancement; and the % relative, which was determined to be peak signal intensity/baseline signal intensity. All of these parameters were measured using a Matlab-based in-house software.
In the eight mice in the treatment group, treatment response was classified as treatment failure, growth retardation and tumor regression. The tumor volume change between day 0 and day 25 was used as a criterion for determining the treatment response. First, the tumor growth rate in the control group was calculated between days 0 and 25 according to the following formula:
where Volday 25 was tumor volume on day 25 and Volday 0 was tumor volume on day 0. The 95% confidence interval of tumor growth rate in the control group was calculated according to the formula
When the tumor volume reduced between days 0 and 25, tumor regression was indicated, where as tumor growth was progressed between days 0 and 25 but the growth rate was less than the lower end of 95% CI of the control group, the growth retardation was considered. Finally, when the tumor growth rate was greater than the lower border of 95% CI of the control group, treatment failure was assumed.
The initial tumor volume was compared to control, treatment failure, tumor regression and growth retardation groups using the Kruskal-Wallis test.
The correlation was evaluated between the initial tumor volume and perfusion parameters and between changes of tumor volume and perfusion parameters, using the Pearson correlation analysis. The strength of the correlation was indicated as follows: 1) strong correlation when the absolute value of correlation coefficient, r was same or greater than 0.8; 2) moderate correlation when the absolute value of r was between 0.5 and 0.8; 3) weak correlation when the absolute value of r was between 0.2 and 0.5; 4) no correlation when the absolute value of r was less than 0.2 (11). In this evaluation, as multiple observations were performed in each subject, Bonferoni correlation was applied to evaluate the statistical significance.
During the follow-up period, one mouse in the control group was deceased, and excluded from data analysis. Therefore, a total of 11 mice, including three mice in the control group and eight mice in the treatment group, were included in the final data analysis.
There was no tumor necrosis in any of the mouse studied as the entire tumor area showed contrast-enhancement on DCE-MR images and the ROIs covered the entire tumor volume for volume measurement.
All mice in the control group showed the mean growth rate of 129% (SD, 28%; range, 97-148%). The lower end of 95% CI for the tumor growth rate in the control group was 74%, which was used for determining the treatment response. In eight mice with sunitinib treatment, four were included in the tumor regression group, of which tumor volume had decreased between days 0 and 25 (mean growth rate, -33%; SD, 11%; range, -59 - -33%). The other four mice in the treatment group showed tumor growth rate less than 79% (mean, 47%; SD, 15%; range, 33-68%) between days 0 and 25, and were therefore, included in the growth retardation group. No mouse showed treatment failure. Accordingly, 11 mice were divided into control group (n = 3), tumor regression group (n = 4), and growth retardation group (n = 4). One mouse in the regression group showed complete tumor regression as there was no residual or recurrent tumor on days 15 and 25.
In the control group, all mice showed continuous tumor growth during all follow-up periods. In the tumor regression group, all mice showed decreased or stable tumor volume in each follow-up period. In the growth retardation group, 20% or greater tumor growth rate was noted between days 15 and 25 (n = 3) and between days 8 and 15 (n = 1).
The mean initial tumor volume was not significantly different in the control, tumor regression and growth retardation groups (p = 0.91) as it was 583 mm3 ± 235 (range, 320-787 mm3) in the control group, 519 mm3 ± 158 in the tumor regression group (311-694 mm3) and 467 mm3 ± 298 in the growth retardation group (226-887 mm3), respectively.
The correlation between the initial tumor volume and perfusion parameter is described in Table 1. Kep (r = 0.80) and initial slope (r = 0.84) showed strong positive correlation to the initial tumor volume (p < 0.01). The time to peak showed moderately negative correlation while AH and Kel showed moderately positive correlation with the initial tumor volume.
The correlation between changes of tumor volume and each perfusion parameter is summarized in Table 2. In all mice, Kep, per cent relative enhancement and initial slope showed significant correlation as the correlation was moderate in Kep and initial slope but weak in per cent relative enhancement. In the subgroup analysis, Kep (r, 0.69-0.81) and initial slope (0.65-0.84) also showed significant correlation in control, tumor regression and growth retardation groups as shown in Figure 1. However, per cent relative enhancement showed significant correlation in the control and tumor regression group but did not depict significance in the growth retardation group. The other parameters, including time to peak, AH and Kel, showed no significant correlation with tumor volume change.
The growth retardation group showed tumor re-growth, the Kep and initial slope rebounded during the follow-up periods. Kep and initial slope increased 20% or greater at earlier (n = 2) or same periods (n = 2) when the tumor started to re-grow with 20% or greater growth rate (Fig. 2). In the early follow-up period after sunitinib administration such as days 0 and 2, 10% or greater decrease of Kep was noted in one (13%) of eight mice, that of per cent relative enhancement in three (38%), and that of initial slope in five (53%) mice.
In this longitudinal study, Kep and initial slope showed significant correlations with the initial tumor volume and also changes in tumor volume. As our tumor model depicts strong VEGFR activity with no significant tumor necrosis, the change in tumor volume indirectly indicate angiogenesis and significant correlation of Kep and initial slope to tumor volume suggest that these two perfusion parameters can be used as adequate surrogate markers for the monitoring of antiangiogenic treatment. Kep is the efflux rate constant from extravascular extracellular space to plasma, therefore indicating the vascular permeability (10). Initial slope indicates the velocity of contrast enhancement in the first-pass extraction, which is dependent on both blood flow and permeability. Earlier reports have indicated that these parameters were accurate and reliable surrogates for evaluating the tumor vascularity and to be important prognostic factors for patient prognosis in breast and ovarian cancers (12, 13).
In the present study, four mice in the growth retardation group showed tumor re-growth during the follow-up period. The Kep and initial slope changed prior to tumor re-growth (n = 2) or at the same period of tumor re-growth (n = 2) suggesting that the Kep and initial slope have a potential to change precedes the change in tumor volume predicts the tumor response in an early stage. This potential may be the most important advantage of DCE-MR in the clinical management of antiangiogenic treatment.
Previous studies showed rapid changes of permeability one or two days following the initiation of antiangiogenic treatment. Based on those results, they suggested that permeability-related parameters, such as Kep can be used as a biomarker for predicting the final outcome of anticancer treatment (14, 15). However, our data do not support the previous studies as the frequency of early decrease in Kep and initial slope was not significant. The discrepancy between previous and our studies imply that the change of perfusion parameters in early stage may vary in early treatment period according to the characteristics of tumor behavior and mechanism of action of the antiangiogenic agent. Therefore, the decision of treatment results on the basis of early change may be inaccurate in some situations. We suggest that the application of DCE-MR should be focused on monitoring the dynamic tumor response after treatment rather than on predicting the final outcome based on their early change.
There are two types of vascular targeting agents. One is a small-molecular receptor tyrosine-kinase inhibitor, such as sunitinib used in this study. The other is a monoclonal antibody against vascular endothelial growth factor, such as bevacizumab. Our study showed that Kep and initial slope were adequate biomarkers for monitoring the effect of sunitinib. Because various types of vascular targeting agents commonly lead to selective inhibition of new vessels and decreased vascular permeability in the tumor, we suggest that Kep and initial slope can be also applied to monitoring the effect of other anti-angiogenic treatment.
To date, several pharmacokinetic models have been introduced by investigators (8, 10, 16). Although the basic concept is shared by these models considering the two compartments for the location of contrast agent, the meaning of extracted parameters and the methods for extracting them are different to some degree. The major characteristic of the Brix model used in our study is no need to measure arterial input function (17). As the arterial input function heavily influences the measurement of perfusion parameters and has been considered to be a main cause of unreliable measurement (18), the basic assumption of this model is the linearity of MR signal intensity and tissue concentration of Gd. Also the concentration of Gd is much lower in tissue (i.e., extravascular space) than in the plasma, the linearity between the signal intensity and Gd concentration is satisfied with our MR acquisition protocol (i.e., TR of 0.02 seconds and TE of 0.00214 seconds) (8, 16). Therefore, to reduce unreliability caused by arterial input function, we adopted Brix pharmacokinetic model which does not require measuring the arterial input function.
For the evaluation of perfusion parameters, the property of contrast agent, particularly the molecular weight, heavily affects measurement (19). Our study used a contrast agent with a low molecular weight, Gd-DOTA (0.76 kD) which has a large first-pass extraction as it rapidly diffuse from the plasma to the extravascular extracellular space. Therefore, the blood perfusion is the dominant factor determining contrast agent kinetic and the permeability approximates to tissue blood flow. Therefore, there is a possibility that Kep was actually influenced by blood flow in our study. Contrarily, with macromolecular contrast material with the molecular weight greater than 30 kD, transport of contrast agent across the vessel wall is the major determining factor of contrast medium kinetics. Hence, macromolecular contrast material may be more adequate than a low-molecular-weight contrast agent for evaluating the vascular permeability of tumor as tumor vessels are known to be more permeable than normal vessels. However, it is still controversial to determine the size of contrast agent to obtaine most reliable and accurate results. Furthermore, protein binding and clumping of high-molecular-weight contrast agents may results in a wide range of molecular diameters. Lastly, the definite limitation of high-molecular-weight contrast agents is that they are not clinically applicable because their safety and pharmacokinetics are not well established.
Our study had no histological validation for perfusion parameters extracted from DCE-MR. Histological correlation was not performed due to microvessel density, a traditional method for evaluating tumor angiogenesis, has shown various strength of correlations with perfusion parameters in previous investigations, some studies found no correlation between perfusion parameters and microvessel density (20-24) and according to Knopp et al. (25), Kep showed no correlation with CD 31 in VEGF-positive breast cancer. Our study performed repeated MR examinations and pathologic examination cannot provide the change of angiogenesis in each mouse. Although controversial results for the correlation between perfusion parameters from DCE-MR and histological results, we believe that the Kep and initial slope can depict the activity of angiogenesis. Actually, microvessel density is not a parameter that determines the dynamic kinetics of contrast agent. Furthermore, in previous studies, accurate pixel-by-pixel comparison of MR image and histological results in the same section plain might have been limited due to technical difficulty.
Small sample size was a limitation of this study is a due to the practical limitations in performing repeated MR examinations (i.e., five times in each tumor mouse in this study), it was difficult to conduct a large-population study. Therefore, our results should be validated in a large-scale clinical study.
In summary, the Kep and initial slope exhibited stronger correlation with initial tumor volume and tumor volume changes after administration of antiangiogenic agent. Hence, the Kep and initial slope may be the accurate and reliable perfusion parameters for monitoring the tumor response to an antiangiogenic treatment.
Figures and Tables
Fig. 1
Tumor volume, Kep and initial slope according to time sequence in mouse with tumor regression by sunitinib treatment.
In this mouse, tumor was not noted in days 15 and 25. A. Tumor volume versus Kep. Kep and tumor volume show similar decrease pattern after sunitinib administration. B. Mean Kep was 0.57/sec in day 0 (left), 0.74/sec in day 2 (center), and 0.24/sec in day 8 (right). C. Tumor volume versus initial slope. Initial slope and tumor volume show similar decrease pattern after sunitinib administration. D. Mean initial slope was 5575/sec in day 0 (left), 5072/sec in day 2 (center), and 1872/sec in day 8 (right).
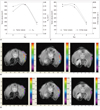
Fig. 2
Tumor volume, Kep and initial slope according to time sequence in mouse with growth retardation by sunitinib treatment.
A. Tumor volume versus Kep. Kep shows earlier rebound than tumor re-growth. B. Tumor volume versus initial slope. Initial slope shows rebound at same time of tumor re-growth.

References
1. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008; 358:2039–2049.
2. Gossmann A, Helbich TH, Kuriyama N, Ostrowitzki S, Roberts TP, Shames DM, et al. Dynamic contrast-enhanced magnetic resonance imaging as a surrogate marker of tumor response to anti-angiogenic therapy in a xenograft model of glioblastoma multiforme. J Magn Reson Imaging. 2002; 15:233–240.
3. Maxwell RJ, Wilson J, Prise VE, Vojnovic B, Rustin GJ, Lodge MA, et al. Evaluation of the anti-vascular effects of combretastatin in rodent tumours by dynamic contrast enhanced MRI. NMR Biomed. 2002; 15:89–98.
4. Rosen MA, Schnall MD. Dynamic contrast-enhanced magnetic resonance imaging for assessing tumor vascularity and vascular effects of targeted therapies in renal cell carcinoma. Clin Cancer Res. 2007; 13(2 Pt 2):770s–776s.
5. Ferl GZ, Port RE. Quantification of antiangiogenic and antivascular drug activity by kinetic analysis of DCE-MRI data. Clin Pharmacol Ther. 2012; 92:118–124.
6. Potapova O, Laird AD, Nannini MA, Barone A, Li G, Moss KG, et al. Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Mol Cancer Ther. 2006; 5:1280–1289.
7. Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003; 9:327–337.
8. Hoffmann U, Brix G, Knopp MV, Hess T, Lorenz WJ. Pharmacokinetic mapping of the breast: a new method for dynamic MR mammography. Magn Reson Med. 1995; 33:506–514.
9. Buckley DL, Kerslake RW, Blackband SJ, Horsman A. Quantitative analysis of multi-slice Gd-DTPA enhanced dynamic MR images using an automated simplex minimization procedure. Magn Reson Med. 1994; 32:646–651.
10. Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997; 7:91–101.
11. Zou KH, Tuncali K, Silverman SG. Correlation and simple linear regression. Radiology. 2003; 227:617–622.
12. Muruganandham M, Lupu M, Dyke JP, Matei C, Linn M, Packman K, et al. Preclinical evaluation of tumor microvascular response to a novel antiangiogenic/antitumor agent RO0281501 by dynamic contrast-enhanced MRI at 1.5 T. Mol Cancer Ther. 2006; 5:1950–1957.
13. Pickles MD, Manton DJ, Lowry M, Turnbull LW. Prognostic value of pre-treatment DCE-MRI parameters in predicting disease free and overall survival for breast cancer patients undergoing neoadjuvant chemotherapy. Eur J Radiol. 2009; 71:498–505.
14. Elsayed YA, Sausville EA. Selected novel anticancer treatments targeting cell signaling proteins. Oncologist. 2001; 6:517–537.
15. Morgan B, Utting JF, Higginson A, Thomas AL, Steward WP, Horsfield MA. A simple, reproducible method for monitoring the treatment of tumours using dynamic contrast-enhanced MR imaging. Br J Cancer. 2006; 94:1420–1427.
16. Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ. Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr. 1991; 15:621–628.
17. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999; 10:223–232.
18. Evelhoch J, Garwood M, Vigneron D, Knopp M, Sullivan D, Menkens A, et al. Expanding the use of magnetic resonance in the assessment of tumor response to therapy: workshop report. Cancer Res. 2005; 65:7041–7044.
19. de Lussanet QG, Langereis S, Beets-Tan RG, van Genderen MH, Griffioen AW, van Engelshoven JM, et al. Dynamic contrast-enhanced MR imaging kinetic parameters and molecular weight of dendritic contrast agents in tumor angiogenesis in mice. Radiology. 2005; 235:65–72.
20. Buckley DL, Drew PJ, Mussurakis S, Monson JR, Horsman A. Microvessel density of invasive breast cancer assessed by dynamic Gd-DTPA enhanced MRI. J Magn Reson Imaging. 1997; 7:461–464.
21. Cooper RA, Carrington BM, Loncaster JA, Todd SM, Davidson SE, Logue JP, et al. Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol. 2000; 57:53–59.
22. Ahn SJ, An CS, Koom WS, Song HT, Suh JS. Correlations of 3T DCE-MRI quantitative parameters with microvessel density in a human-colorectal-cancer xenograft mouse model. Korean J Radiol. 2011; 12:722–730.
23. Kang H, Lee HY, Lee KS, Kim JH. Imaging-based tumor treatment response evaluation: review of conventional, new, and emerging concepts. Korean J Radiol. 2012; 13:371–390.
24. Kim JK, Jang YJ, Cho G. Multidisciplinary functional MR imaging for prostate cancer. Korean J Radiol. 2009; 10:535–551.
25. Knopp MV, Weiss E, Sinn HP, Mattern J, Junkermann H, Radeleff J, et al. Pathophysiologic basis of contrast enhancement in breast tumors. J Magn Reson Imaging. 1999; 10:260–266.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


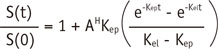


 XML Download
XML Download