Abstract
Metastases to intramammary nodes have been shown to be an independent predictor of poor outcome in patients with breast cancer, such as axillary lymph node metastases. The detection and accurate characterization of these nodes preoperatively is thus crucial for the staging and planning of treatment for breast carcinoma, particularly in cases with axillary lymph node negative disease as it upgrades the disease staging. We herein report the first case where we detected an intra-mammary node on specimen MRI after the primary pathological gross specimen evaluation failed to detect the node.
Intramammary nodes are frequently diagnosed preoperatively on various imaging modalities in patients with breast carcinoma. We herein discuss a case where an intramammary node in a case of axillary node negative carcinoma breast was localized using post-mastectomy specimen MRI and was proven to be malignant; hence, it upstaged the disease and even altered the management. We intend to highlight the importance of these often neglected nodes and to emphasize the need of scrupulous radiological and pathological examinations in detecting these nodes, and thereby improve the management of patients suffering from breast carcinoma.
A 61-year-old female was presented to the breast clinic with complaints of a lump in the right breast since the past 3 months. On examination, a lump was palpated in the right upper-outer quadrant without any palpable axillary nodes. The contralateral breast and axilla were clear. Mammography showed a high density lesion in the upper outer quadrant of the right breast with perilesional architectural distortion and spiculations (Fig. 1A). The MRI showed a soft tissue mass showing irregular spiculated margins in the upper outer quadrant of the right breast which was hypointense on T1W images and hyperintense on Short Tau (t) (inversion time) Inversion Recovery (STIR) images; further, it showed areas of restricted diffusion at the periphery. Another similar appearing nodular lesion was noted at the right 12 o'clock position measuring approximately 5 mm (Fig. 1B-E). It showed a homogeneous contrast enhancement with rapid increase in signal intensity on the postcontrast scan, followed by a washout-type II signal intensity curve favoring a malignant lesion. A second-look ultrasound examination was performed, presenting a tiny hypoechoic nodule (Fig. 1F). With the lesion being small, an ultrasound-guided FNAC was attempted in the same sitting, which was negative for malignancy. The lesion was classified as indeterminate and the patient underwent modified right radical mastectomy with axillary node sampling. Sentinel lymph node biopsy and lymphoscintigraphy are not routinely performed at out institution. The final histopathology was reported as infiltrating ductal carcinoma; grade II without nodal involvement as the axillary nodes were negative. The smaller lesion was not evaluated in pathological grossing owing to its size. As the lesion was highly suspicious for malignancy, the resected pathology specimen was subjected to an MRI examination .The specimen MRI confirmed the STIR hyperintense lesion observed previously (Fig. 1G). The lesion was then localized under MR guidance and methylene blue was injected at the site (Fig. 1H). The specimen was sent back for regrossing and pathology examination. Additional sections were taken from the marked region, which showed intramammary node with metastasis from ductal carcinoma without perinodal extension (Fig. 1I). Thus, the disease was then labeled as follows: carcinoma with axillary node-negative but metastatic intramammary node, which upgraded the disease to stage II. The patient was placed on adjuvant systemic therapy and close follow-up, as the presence of intramammary node correlates with poorer overall prognosis.
Development of distant metastases is the major cause of mortality and morbidity in patients with breast cancer. Axillary node metastases have proven to be the most important prognostic indicator, and are significantly associated with decreased disease free survival and overall survival (1). Intramammary nodes have been detected on pre-operative imaging modalities, such as mammography and USG, during surgery and post-operatively in the dissected specimens. However, the clinical significance of a metastatic intramammary node is not well established.
Intramammary lymph nodes (IMLN) have been reported in approximately 5% of patients undergoing mammography (2). Although they can be seen in any quadrant, they are most commonly located in the upper outer quadrant (3). Intramammary lymph node should be completely surrounded by breast parenchyma, a criteria which helps to distinguish them from inferior axillary nodes which also overlie the pectoral muscles region on mammography (4). Intramammary lymph nodes are considered normal if they have the following characteristics: well-defined, round to oval moderate density, contain a radiolucent fatty hilum and measure less than 1 cm (5). On ultrasound, benign intramammary node appears as a ovoid, isoechoic or slightly hypoechoic nodule with an echogenic fatty hilum (5, 6). The sensitivity of our current imaging method in the detection of intramammary node metastases is low (7). The reported incidence of IMLNs varies widely from less than 1% to almost 50%. This wide variability depends on the method of identification, (radiographic, surgical or pathological reporting) and how meticulously the pathology specimens were scrutinized (8). For example, in the technique used by McSweeney and Egan (9), the breast specimens were sliced into 5 mm-thick sections and IMLN were identified in 29% of breasts with carcinoma and in 47% of benign breasts. It has been shown that scrupulous pathological analysis is superior to imaging studies, lymphoscintigraphy or retrospective database reviews at identifying IMLNs (8). In our case, however, the node was detected on imaging and was missed on pathology examination.
The therapeutic significance of positive IMLNs is not yet well-established. These are the potential sites for regional spread from breast cancer; moreover, metastases to these sites have been reported up to 9.8% of operable breast cancer (1, 9, 10). IMLN does not find a separate mention in the current AJCC staging manual for breast cancer and a metastatic IMLN is considered equivalent to an axillary node and thus, labels the disease as N1 (11). The presence of metastatic IMLN correlated with poorer prognosis in patients with stage I disease, whereas it had no effect on the prognosis of patients with stage II disease (9). Various studies have established that intramammary lymph node metastases are an independent predictor of poor outcome (poor disease-free survival, disease-specific survival and overall survival) in patients with breast carcinoma compared with intramammary negative patients (1, 7, 12). The issue which needs attention is the case wherein there are isolated metastases to the IMLN with negative axillary metastases. Guth et al. (10) in their study have reiterated the finding that patients with positive IMLNs have more aggressive cancer, with higher grade and stage, higher rates of lymphovascular invasion and axillary nodal disease and more frequent multifocal disease; hence, their presence mandates axillary dissection and influences adjuvant therapy. Further, Hogan et al. (1) report a case wherein a patient had a benign axillary sentinel lymph node with metastatic intramammary lymph node; hence, axillary dissection was deferred. However the patient developed a recurrence in the axilla later. In addition, intramammary lymph node metastases are a greater predictor of additional nodal disease than a metastatic axillary sentinel lymph biopsy (1).
Thus, the presence of IMLNs has important clinical implications. It is essential to detect these on pre-operative imaging and to evaluate them by histopathological examination. Post-operatively, the excised breast specimens should be carefully examined in order to detect the presence of metastatic intramammary nodes. The radiologist should communicate the imaging findings to the pathologist so that these smaller nodes are also accurately characterized. Post-operative specimen USG or MRI can be a useful aid to guide the pathologist in cases where there is a high index of suspicion and the routine specimen examination has failed to detect these nodes.
The detection and accurate characterization of IMLNs nodes preoperatively is crucial for the staging and planning of treatment for breast carcinoma, particularly in cases with axillary lymph node negative disease as it upgrades the disease and warrants further axillary dissection; hence, such patients then become candidates for aggressive adjuvant systemic therapy. It is important not only for clinicians but also for radiologists and pathologists to be aware of the prognostic implications associated with these nodes in order to ensure prompt diagnosis. A good collaboration between the three can ensure higher detection of these often neglected nodes and ultimately improve patient care.
Figures and Tables
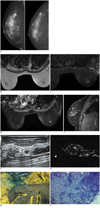 | Fig. 1Digital mammography (A), magnetic resonance imaging of the breast (B-E), targetted USG (F), post opearative specimen MRI (G), photograph of resected specimen (H), and photomicrograph from obtained after localisation (I).
A. Mammogram of right breast shows mixed fibrofatty and glandular parenchyma with high density mass in upper outer quadrant of right breast with perilesional architectural distortion and spiculations measuring approximately 1.3 × 1.1 cm. Centimeter sized axillary node is also noted. Other than these, no other suspicious lesion is noted. Left breast was normal.
B-E. Precontrast axial T1W depicting tiny hypointense nodule noted at 12 o'clock position in right breast (B) which appears hyperintense on precontrast (C). Postcontrast T1W axial (D) and sagittal images (E) of breast showing nodule in right breast. STIR = Short Tau Inversion Recovery, T1W = T1 weighted
F. Ultrasonography of right breast showing tiny hypoechoic nodule with rim of hyperechoic tissue in 12 o'clock position. FNAC was performed in same setting, which was negative for malignancy. G. STIR MRI sequence of resected breast specimen shows hyperintense nodule in specimen. H. Photograph of resected specimen with methylene blue injected at suspicious site, which was localized using STIR MRI sequence. I. Photomicrograph obtained from site after localization of lesion using methylene blue shows metastatic ductal carcinoma cells along sinuses (HFE: 100). STIR = Short Tau Inversion Recovery,
|
References
1. Hogan BV, Peter MB, Shenoy H, Horgan K, Shaaban A. Intramammary lymph node metastasis predicts poorer survival in breast cancer patients. Surg Oncol. 2010; 19:11–16.
2. Stomper PC, Leibowich S, Meyer JE. The prevalence and distribution of well circumscribed nodules on screening mammography: analysis of 1500 mammograms. Breast Dis. 1991; 4:197–203.
3. Svane G, Franzén S. Radiologic appearance of nonpalpable intramammary lymph nodes. Acta Radiol. 1993; 34:577–580.
4. Egan RL. Breast imaging: diagnosis and morphology of breast diseases. Philadelphia, Pa: Saunders;1988. p. 313–323.
5. Lindfors KK, Kopans DB, Googe PB, McCarthy KA, Koerner FC, Meyer JE. Breast cancer metastasis to intramammary lymph nodes. AJR Am J Roentgenol. 1986; 146:133–136.
6. Lee CH, Giurescu ME, Philpotts LE, Horvath LJ, Tocino I. Clinical importance of unilaterally enlarging lymph nodes on otherwise normal mammograms. Radiology. 1997; 203:329–334.
7. Shen J, Hunt KK, Mirza NQ, Krishnamurthy S, Singletary SE, Kuerer HM, et al. Intramammary lymph node metastases are an independent predictor of poor outcome in patients with breast carcinoma. Cancer. 2004; 101:1330–1337.
8. Diaz R, Degnim AC, Boughey JC, Nassar A, Jakub JW. A positive intramammary lymph node does not mandate a complete axillary node dissection. Am J Surg. 2012; 203:151–155.
9. McSweeney MB, Egan RL. Prognosis of breast cancer related to intramammary lymph nodes. Recent Results Cancer Res. 1984; 90:166–172.
10. Guth AA, Mercado C, Roses DF, Hiotis K, Skinner K, Diflo T, et al. Intramammary lymph nodes and breast cancer: a marker for disease severity, or just another lymph node? Am J Surg. 2006; 192:502–505.
11. Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin. 2006; 56:37–47. quiz 50-51.
12. Nassar A, Cohen C, Cotsonis G, Carlson G. Significance of intramammary lymph nodes in the staging of breast cancer: correlation with tumor characteristics and outcome. Breast J. 2008; 14:147–152.
13. Pugliese MS, Stempel MM, Cody HS 3rd, Morrow M, Gemignani ML. Surgical management of the axilla: do intramammary nodes matter? Am J Surg. 2009; 198:532–537.
14. Intra M, Garcia-Etienne CA, Renne G, Trifirò G, Rotmensz N, Gentilini OD, et al. When sentinel lymph node is intramammary. Ann Surg Oncol. 2008; 15:1304–1308.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download