Abstract
Langerhans cell sarcoma (LCS) is a neoplastic proliferation of Langerhans cells with malignant cytological features and multi-organ involvement that typically has a poor prognosis. We experienced 2 cases of LCS in children less than 2 years of age and report them based primarily on CT and MR findings. Both children had findings of hepatosplenomegaly with low-attenuation nodular lesions, had multiple lymphadenopathy, and had shown recurrent lesions invading the skull during follow-up after chemotherapy.
Langerhans cells are a subset of antigen-presenting dendritic cells belonging to the histiocytic system. According to the World Health Organization, there are 2 types of Langerhans cell tumors: Langerhans cell histiocytosis (LCH) and Langerhans cell sarcoma (LCS). LCH is proliferating disorder of Langerhans cells, while LCS is a neoplastic proliferation of Langerhans cells showing malignant cytological features (1). LCS can develop de novo or progress from LCH (2).
Langerhans cell sarcoma is very rare, and because it shows similarities to LCH, it is diagnosed based on malignant cytological features. Its characteristic clinical manifestations and image findings remain unknown. LCS may develop in any age group, but few cases have been reported in patients aged 10 or younger (3, 4). In the present study, we report 2 cases of LCS based on CT and MR findings that developed in children younger than 2-years-old.
An 11-month-old female infant was admitted to our hospital with a chief complaint of intermittent fever that had persisted for the prior month. The patient was born at term during an uneventful delivery and had no medical problems. Physical examination revealed splenomegaly and multiple petechiae in both inguinal areas. On blood testing there were findings of anemia and thrombocytopenia. No specific findings were observed during a bone marrow examination. Contrast-enhanced abdominal CT and chest CT showed hepatosplenomegaly (spleen length: 14 cm), and irregular, multifocal low-density lesions in the spleen (Fig. 1A). Mild enlargements of the lymph nodes were found in the cervical, mediastinal, and pulmonary hilar regions, and the in the porta hepatis. Small amounts of bilateral pleural effusions and ascites were also observed (Fig. 1B).
Since the patient's fever did not subside after treatment with antibiotics and steroids, a splenectomy and multiple mesenteric lymph node biopsies were performed. LCS was confirmed by immunohistochemical study, as tumor cells were positive for CD1a and S-100 with malignant cytological features (Fig. 1C-E). The patient was started on chemotherapy with etoposide and dexamethasone based on the hemophagocytic lymphohistiocytosis (HLH) 2004 protocol. On follow-up, a whole-body MRI obtained 10 months after chemotherapy, the only remaining findings were mild hepatomegaly; and the multiple lymph node enlargements had disappeared.
A contrast-enhanced orbit CT and MRI conducted 6 months later as a result of a right orbital swelling, found a well-enhancing soft tissue mass invading the anterior skull base and both orbital areas, accompanied by bony destruction with an irregular, but clear boundary. Neither periosteal reaction nor calcification was observed inside the mass (Fig. 1F, G). Biopsy confirmed the recurrence of LCS with positive CD1a and S-100. Treatment for recurrent LCS was given with 3 cycles of ifosfamide, carboplatin, and etoposide.
On a follow-up brain MRI obtained after 2 months, the skull lesion showed complete response. We are now following the patient after performing bone marrow transplantation.
A 17-month-old girl visited our hospital with a chief complaint of intermittent fever that had persisted for the past month, abdominal pain and poor appetite. She was born via normal vaginal delivery with a birth weight of 3.0 kg and had an unremarkable past medical and family histories. Physical examination revealed hepatosplenomegaly and multiple petechiae of the scalp and the lower abdomen. Anemia and thrombocytopenia were noted on blood testing. Upon bone marrow examination, invasion of histiocytic neoplasm was observed.
Contrast-enhanced abdominal CT showed hepatosplenomegaly, periportal low density and multiple small, hypodense-lesions in the liver. Conglomerated lymph node enlargement was found in the porta hepatis, and a huge and heterogeneously enhancing soft tissue mass was observed in the anterior mediastinum (Fig. 2A, B). A biopsy of the mass was conducted extending up to the right supraclavicular region, the scalp, and the abdominal skin lesion. LCS was diagnosed by the malignant cytological features in the lesions, and immunohistochemistry tests that were positive for CD1a, S-100, and CD68. On a follow-up whole-body MRI conducted one year after chemotherapy based on HLH 2004 protocol, the mediastinal mass and lymphadenopathy around the porta hepatis had decreased in size. After 5 months, recurrence on the scalp was suspected, and contrast-enhanced brain MRI and skull base CT showed multifocal osteolytic lesions and enhancing soft tissue lesions in both temporal regions (Fig. 2C, D). A biopsy of the scalp lesion was conducted, and based on positive findings for CD1a and S-100, recurrence of LCS was confirmed before resuming chemotherapy. The patient was treated with 2-CdA and Ara-C combination therapy based on LCH-S-2005 protocol. On follow-up brain MRI obtained one year after treatment, the skull lesion showed improvement. However, on serial ultrasounds of the liver and contrast-enhanced abdominal CT, the volume of the right hepatic lobe gradually decreased, and the caudate lobe and the left hepatic lobe showed hypertrophic cirrhotic morphology. In addition, scattered nodular calcifications were newly formed in the liver parenchyma, and gradually increased in number, spreading along the intrahepatic biliary trees (Fig. 2E, F). The patient is now being followed-up after bone marrow transplantation.
Langerhans cell sarcoma is a very rare disease, but according to precedent studies, can develop in any age group, and is slightly more common in females than in males (1). LCS can invade multiple systems or tissues-bone, lung, brain, skin/mucous membranes, lymph nodes, liver and many other soft tissues. LCS grows fast, showing aggressive clinical behaviors, resulting in a poor prognosis and short survival period (1). Due to the paucity of reported cases, no characteristic image findings and established method of treatment are known (4). Most reported cases of LCS focus mainly on pathologic findings.
The diagnosis of LCS can be made based on malignant cytological features such as atypia, hyperchromatic nuclei, prominent nucleoli, and frequent mitotic figures, and typical immunophenotype findings such as expression of CD1a, S-100 protein, and Langerin (CD207). In addition, typical Birbeck granules may be seen, and expressions of various histiocytic markers such as CD68 are also frequently observed (3).
Researchers and clinicians have treated patients with LCS in accordance with the common lymphoma treatment protocols using cyclophosphamide, prednisolone, adriamycin, and vincristine, but there is no generally accepted optimal treatment regimen. Uchida et al. (5) reported the successful treatment by mesna, doxorubicin, ifosfamide, and dacarbazine regimen, which is one of the treatment protocols for soft tissue sarcoma. In LCH patients, different treatment strategies including chemotherapy, surgery, or radiation therapy have been employed depending on the site and extent of the disease and the clinical course. Considering that LCS behaves in a very malignant fashion with a poor outcome, a more aggressive treatment approach is necessary for patients with LCS, and it is, therefore, important to distinguish LCS from LCH. The 2 children described in the present report were treated with regimens proposed as effective agents for refractory multisystem LCH and HLH (6, 7).
Our patients are the youngest patients ever described in the English language medical literature (3, 4). In the initial work ups, both patients showed hepatomegaly or splenomegaly accompanied by enlargement of multiple lymph nodes. Lymph nodes were conglomerated, showed heterogeneous enhancement, and encased the surrounding vessels without vascular obstruction. Liver and spleen parenchyma showed multifocal low density lesions. In the present cases, the most common differential diagnoses clinically included hematologic malignancy or lymphoproliferative diseases, such as non-Hodgkin lymphoma, HLH and Epstein-Barr Virus infection. LCS cannot be differentiated from these diseases by imaging findings alone.
On follow-up imaging tests conducted after chemotherapy, multiple enlarged lymph nodes showed significant reductions in size and in number, and were almost imperceptible. In contrast, hepatosplenomegaly did not improve.
In cases of liver involvement of LCH, periportal low density can be seen in CT images due to periportal histiocyte infiltration, inflammation, and edema. In addition, sclerosing cholangitis, a known late complication, and biliary cirrhosis accompanied by biliary wall calcification may also develop (8). Splenic involvement is known to manifest as splenomegaly and multiple low-density lesions on contrast-enhanced CT images (9). These image findings may be seen in cases of LCS due to the similar pathophysiology. However, unlike LCH, in which punctate, calcification in the thymus has been described, thymic calcification could not be seen in our 2 patients (10).
Upon interruption of chemotherapy, recurrences of soft tissue masses in the skull base and around the orbit were observed in both patients. These recurrent lesions showed iso- to hypointense signals on T2-weighted MR images and heterogeneous enhancement patterns, accompanied by destruction of adjacent bony structures and displacement of orbits. These findings are also similar to the findings of orbital involvement in LCH (11).
In conclusion, LCS can develop in children younger than 2-years-old, and in cases of nonspecific hepatosplenomegaly with multifocal low density lesions and multiple conglomerated lymphadenopathy, LCS should be included in the differential diagnosis. Biopsy is absolutely required for the diagnosis of LCS, because similar image findings may also be observed in LCH and the choice of therapeutic regimen varies. In addition, since recurrence may manifest as brain and skull lesions during follow-up, head and neck imaging tests should be conducted on a regular basis.
Figures and Tables
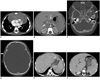
Fig. 1
Case 1. 11-month-old female infant presented with intermittent fever.
A. Contrast-enhanced CT of abdomen shows splenomegaly (about 14 cm) with multifocal low density lesions in spleen (arrows). Soft tissue density lesions are seen in porta hepatis (arrowheads). B. Contrast-enhanced CT of chest shows nodular enlargement of thymus with bilateral pleural effusions. C. Typical histology of Langerhans cell sarcoma with malignant cytological features, such as atypia, hyperchromatic nuclei (H&E, × 400), and prominent nucleoli. D, E. Cells are immunopositive for CD1a (× 400) and S-100 (× 400). F. At 16 months after chemotherapy, follow-up MRI of orbit (fat-suppressed T2-weighted image) shows well-enhancing soft tissue mass extending from anterior skull base (including sphenoid wing and infratemporal fossa) to lateral aspect of right orbit and frontal area (arrow). G. Follow-up CT of orbit shows associated adjacent bony destruction with irregular sharp margin (arrow). Osteolytic lesions involving anterior skull base to lateral aspect of left orbit are also noted (arrowheads).

Fig. 2
Case 2. 17-month-old girl presented with intermittent fever.
A. Contrast-enhanced CT of abdomen shows huge soft tissue density lesion with heterogeneous enhancement in anterior mediastinum. B. In upper abdomen, soft tissue density lesions encase main portal vein, hepatic artery and splenic artery, without evidence of luminal narrowing (arrows). C, D. At 17 months after chemotherapy, follow-up CT of skull shows multifocal osteolytic lesions including greater wing of left sphenoid bone, and right temporal bone (arrows). E, F. One year after salvage chemotherapy, follow-up contrast-enhanced CT of abdomen shows nodular calcifications along biliary trees.
References
1. Zhao G, Luo M, Wu ZY, Liu Q, Zhang B, Gao RL, et al. Langerhans cell sarcoma involving gallbladder and peritoneal lymph nodes: a case report. Int J Surg Pathol. 2009. 17:347–353.
2. Lee JS, Ko GH, Kim HC, Jang IS, Jeon KN, Lee JH. Langerhans cell sarcoma arising from Langerhans cell histiocytosis: a case report. J Korean Med Sci. 2006. 21:577–580.
3. Nakayama M, Takahashi K, Hori M, Okumura T, Saito M, Yamakawa M, et al. Langerhans cell sarcoma of the cervical lymph node: a case report and literature review. Auris Nasus Larynx. 2010. 37:750–753.
4. Luo B, Pian B, Peng Z, Jintao D, Shixi L. Pharyngeal tonsil manifestation of Langerhans cells sarcoma: a case report and review of the literature. Int J Pediatr Otorhinolaryngol Extra. 2011. 6:156–158.
5. Uchida K, Kobayashi S, Inukai T, Noriki S, Imamura Y, Nakajima H, et al. Langerhans cell sarcoma emanating from the upper arm skin: successful treatment by MAID regimen. J Orthop Sci. 2008. 13:89–93.
6. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007. 48:124–131.
7. Donadieu J, Chalard F, Jeziorski E. Medical management of langerhans cell histiocytosis from diagnosis to treatment. Expert Opin Pharmacother. 2012. 13:1309–1322.
8. Caruso S, Miraglia R, Maruzzelli L, Luca A, Gridelli B. Biliary wall calcification in Langerhans cell histiocytosis: report of two cases. Pediatr Radiol. 2008. 38:791–794.
9. Pyun HW, Kim ME, Kim JH. CT and MR findings of langerhans cell histiocytois involving the spleen: a case report. J Korean Radiol Soc. 2002. 46:171–174.
10. Heller GD, Haller JO, Berdon WE, Sane S, Kleinman PK. Punctate thymic calcification in infants with untreated Langerhans' cell histiocytosis: report of four new cases. Pediatr Radiol. 1999. 29:813–815.
11. Yi G, Yoon HK, Kim BK, Kim KA, Choo IW. CT findings of orbital langerhans cell histiocytosis. J Korean Radiol Soc. 2000. 42:841–846.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download