Abstract
Objective
The purpose of this study was to establish a minimally invasive and reproducible protocol for estimating the gastrointestinal (GI) transit time in mice using barium and radiopaque markers.
Materials and Methods
Twenty 5- to 6-week-old Balb/C female mice weighing 19-21 g were used. The animals were divided into three groups: two groups that received loperamide and a control group. The control group (n = 10) animals were administered physiological saline (1.5 mL/kg) orally. The loperamide group I (n = 10) and group II (n = 10) animals were administered 5 mg/kg and 10 mg/kg loperamide orally, respectively. Thirty minutes after receiving the saline or loperamide, the mice was administered 80 µL of barium solution and six iron balls (0.5 mm) via the mouth and the upper esophagus by gavage, respectively. Afterwards, the mice were continuously monitored with fluoroscopic imaging in order to evaluate the swallowing of the barium solution and markers. Serial fluoroscopic images were obtained at 5- or 10-min intervals until all markers had been excreted from the anal canal. For analysis, the GI transit times were subdivided into intestinal transit times (ITTs) and colon transit times (CTTs).
Results
The mean ITT was significantly longer in the loperamide groups than in the control group (p < 0.05). The mean ITT in loperamide group II (174.5 ± 32.3) was significantly longer than in loperamide group I (133.2 ± 24.2 minute) (p < 0.05). The mean CTT was significantly longer in loperamide group II than in the control group (p < 0.05). Also, no animal succumbed to death after the experimental procedure.
Gastrointestinal (GI) disorders are common reasons for patient visits, and GI discomfort and disorders are important health concerns (1). Therefore, researchers must be able to identify and evaluate commonly available natural drugs. These drugs are potential alternatives to stimulatory or inhibitory drugs. Normal GI transit time, especially those related to small intestine and colon transit times (CTTs), is used to diagnose GI motility disorders. Thus, researchers and clinicians should determine the GI motility of animal models in order to develop further alternatives. Small rodents are most commonly used as animal models, and many methods of assessing GI transit time have been described (2-11).
Gamma scintigraphy using orally administered radioactive pellets provides a less-invasive, physiologic, accurate, repeatable method. However, it is expensive, time consuming, and is limited by the use of radioisotopes (2-6). Barium sulphate suspensions are often used to determine gastric emptying times. However, this technique is not quantitative and determines times for liquids but not solids (4, 5). Radiopaque markers, such as barium impregnated polyethylene spheres (BIPS) in domestic animals (cats and dogs), are used to characterize the movement of food through different segments of the GI tract. These spheres are available in two sizes (1.5- and 5-mm diameters), which are too large for use in mice (5, 8-10).
An aqueous suspension of charcoal has been widely used to evaluate GI transit times in rodents. Animals are given an aqueous suspension of charcoal after 3-24 hours of food deprivation. Then, the animals are sacrificed after the charcoal is administered. The small intestine is removed in order to determine the percentage traversed by feces containing charcoal. The distance traveled by the charcoal provides a measure of the GI transit time (2, 7, 11-14). However, these studies are invasive and not reproducible or sufficient enough for evaluating GI transit time. The purpose of this study was to establish a minimally invasive and reproducible protocol for estimating the GI transit time in mice through the use of barium and radiopaque markers.
Twenty 5- to 6-week-old Balb/C female mice body weighing 19-21 g were used in this study. The mice were housed in polyethylene cages (30 × 20 × 12 cm) under standard environmental conditions (21 ± 1℃ with a reversed 12 light/dark cycle and relative humidity of 50-60%) for 7 days before the experiment was performed. In addition, the animals were provided a normal commercial diet and were allowed water ab libitum. Animal care, experiments, and euthanasia were performed in accordance with the protocols issued by the Chonnam National University Animal Research Committee.
Metal balls 0.5 mm in diameter (Stainless ball®, Agami Modeling, Gunpo, Korea) were used as radiopaque markers, and coated with methoxy polyethylene glycoldopamine (m-PEG-dopamine), which was synthesized by reacting m-PEG acetic acid (m-PEG-COOH, MW 5000), 3-hydroxytyramine hydrochloride (dopamine), N-(3-dimethylaminopropyl)-N'-ethyl carbodiimide hydrochloride, and N-hydroxysuccinimide (Sigma-Aldrich, St. Louis, MO, USA) for 2 days at room temperature. PEG is biocompatible, and dopamine has been previously used to bio-compatibilize metal surfaces (15).
The metal balls were coated as previously described (15). Briefly, m-PEG-dopamine (40 mg) was dissolved in a borate buffer (10 mM, 10 mL, pH 8.5), and the metal balls (100 EA) were added for 2 days at room temperature. The balls was then washed 2-3 times with distilled water and dried in an oven. As shown in Figure 1, the irregular surfaces of the ball were smoothed by the coating procedure.
All mice were deprived of food for 1 hour before the experimental procedure. The study was designed to include one control and two test groups as follows: the mice in the control group (n = 10) were administered physiological saline (1.5 mL/kg, p.o.) using a 20 G gavage needle; and the mice in loperamide groups I and II (n = 10) were administered a loperamide mixture (5 or 10 mg/kg, respectively, p.o.) using similar gavage needles. Loperamide hydrochloride, an antidiarrheal agent, was purchased as loperamide Cap® from Samnam Pharmaceuticals (Daejeon, Korea) (12, 13). Single capsules of loperamide hydrochloride (2 mg) were dissolved in 300 µL of 0.9% physiological saline.
Thirty minutes after ingesting the solutions, the mice were placed in a plastic induction chamber where general anesthesia was induced using 3% vaporized isoflurane (Forane®, Joong-Wea Pharm, Seoul, Korea) in 100% oxygen. Mild anesthesia was induced within 30-60 seconds. Immediately after general anesthesia was induced, the mice were taken out of the induction chamber, and six iron markers and 80 µL of barium solution were administered directly in the mouth or upper esophagus using a 20 G gavage needle. The mice swallowed the markers and barium while recovering from the mild sedation. The barium solution (Solotop suspension® 70, Taejon Pharm, Seoul, Korea) was dissolved in phosphate buffered saline (1 : 1.25).
Immediately after the gavage procedure, the mice were moved to plastic cages and continuously monitored using a fluoroscopic imaging system (Siemens AXIOM ICONOS R200 3D, Siemens Healthcare System, Erlangen, Germany). Fluoroscopic imaging was performed for 3- or 5 minutes intervals at 50 kVp and 0.6 mAs. The mice were fully conscious during the procedure. Fluoroscopic imaging continued until all markers had been excreted from the anal canal. After the markers and barium solution were administered, the mice were deprived of food for 1 hour but were allowed free access to water. The handling of animals and all experimental procedures were consistent across the three study groups.
Fluoroscopic image analysis was performed through consensus between two radiologists. The anatomical locations of the stomach, small intestine, cecum, and colon were determined based on mouse anatomy (Fig. 2) (16). Mean small intestinal transit times (ITTs) and CTTs were estimated using the marker movements on the fluoroscopic images. The mean ITT was defined as the amount of time taken for three of the six markers to pass from the pylorus to the cecum. The mean CTT was defined as the amount of time taken for the three markers in the cecum to be excreted from the anal canal (4, 5, 8, 9).
According to our protocol, the mice fasted for 1 hour, which allowed the stomach to empty its food contents. Anesthesia was induced with 3% isoflurane inhalation for 30-60 seconds. After recovering from the isoflurane general anesthesia, the mice were immediately administered the metal markers and barium. Barium was used to determine the anatomical location of the GI tracts. On the fluoroscopic images, radiopaque markers were monitored in the stomach, small intestine, cecum, and colon to determine the GI transit times (Fig. 3). No animal was sacrificed after the experimental procedure.
The mean ITT was significantly longer in the loperamide groups than in the control group (p < 0.05). The mean ITT in loperamide group II (174.5 ± 32.3) was significantly longer compared to loperamide group I (133.2 ± 24.2 minute) (p < 0.05). Also, the loperamide groups showed a significant increase in ITT when the loperamide dosage was increased. The mean CTT was significantly greater in loperamide group II than in the control group (p < 0.05). However, the mean CTT in loperamide group I was not significantly different from that in the control group (p = 0.91). The mean CTTs were identical in the two loperamide groups (p = 1.00) (Table 1).
Our data showed that the mean ITTs and CTTs were determined by monitoring the movements of radiopaque markers during GI transit. Iron balls used as radiopaque markers were 0.5 mm in diameter, and, thus, were easily swallowed. To avoid stimulation of the intestinal mucosa, the balls were coated with PEG conjugated to the amine group of dopamine through amide bonding. Recently, a report showed that X-ray imaging can be used for simple visualization and localization of solid dosage forms in rats in the fed state using shortened commercial minicapsules (17). The radiopaque markers in our protocol provided quantitative, real-time assessments of solid food-like transit times. It was not true solid food, but similar to the hard tablets. The radiopaque marker in our protocol was similar to that reported in previous studies, which used radiopaque with BIPS (8-10, 18). However, BIPS were used only in dogs or cats because the spheres were too large to use in the mouse model.
Our protocol allowed the increased GI transit time generated by loperamide to be investigated with fluoroscopic imaging. The mean ITT was significantly longer in the loperamide groups than in the control group. Furthermore, the loperamide groups showed a marked increase in the ITT when the loperamide dosage was increased, and the mean CTT was significantly greater in loperamide group II than in the control group. This significant difference between the mean ITT values of the control and loperamide groups suggests the reliability of our protocol. Loperamide is a peripheral µ-opioid receptor agonist and a well-recognized antidiarrheal agent. In addition, loperamide markedly and dose-dependently inhibits small intestine propulsive motility (19). This explains the differential effects of loperamide on intestinal motility; that is, loperamide inhibits small intestine motility (13).
Our protocol showed that barium sulfate filling the GI tract delineates the intestinal anatomy. However, use of barium sulfate for evaluating GI transit time is limited because of barium's liquid nature. Since gastric pylorus provides little resistance to the passage of liquids, they tend to move quickly from the stomach. In a previous study, barium moved more rapidly in small and large intestines than solids (4, 5).
Isoflurane is the preferred anesthetic for most small and large animal procedures, and provides rapid induction, preserves cardiac output, and maintains a safe level of surgical anesthesia through spontaneous ventilation (20, 21). Isoflurane is usually delivered at 3.5 to 4.5% gas in oxygen to induce anesthesia and at 1.5 to 3% to maintain anesthesia. A previous study investigated the effects of GI motility changes in rats after exposure to isoflurane for general anesthesia. Following the study, the researchers concluded that isoflurane is the anesthetic of choice in intestinal drug absorption studies (14).
Our protocol has several advantages. First, our protocol was carried out on non-stressed animals. Second, the protocol determines actual transit times in the small and large intestine. Third, the protocol is minimally invasive and does not require animal sacrifice, which means that animals can be reused. However, the disadvantages of our protocol include the risk of marker aspiration and the use of ionizing radiation.
In conclusion, our protocol based on the use of radiopaque markers and barium within the mouse model provides a reproducible, minimally invasive means of determining GI transit times, and, thus, is suitable for evaluating GI motility and drug effects in mice.
Figures and Tables
Fig. 1
Scanning electron microscope images showing surface of iron ball.
A. Iron ball before coating reveals black lines and irregular surface. B. Iron ball after surface was coated has changed uniformly.

Fig. 2
Schematic diagram of mouse gastrointestinal anatomy.
E = esophagus, S = stomach, SI = small intestine, Ce = cecum, Co = colon
Fig. 3
Fluoroscopic images showing gastrointestinal (GI) transit of barium sulfate and radiopaque iron balls.
Fluoroscopic images demonstrate: A. Six iron balls (long arrows) in stomach. B. Three markers in proximal small intestine (short arrows). C. Six markers (short arrows) in small intestine. D. Three markers in cecum (long arrows) (other three remained in small intestine [short arrows]). E. All markers in cecum (long arrows). F. All markers in colon (short arrows). G. Three markers eliminated from GI tract (other three are in colon [short arrows]). H. Clearance of all markers via anal canal.
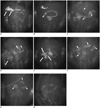
Table 1
Effects of Loperamide on GIT

Note.- Data are means ± standard deviations. *ITT, CTT, and GIT were significantly longer in loperamide group than in control group, †ITT and GTT were significantly longer in loperamide group II than in loperamide group I. ITT = small intestinal transit time, CTT = colon transit time, GIT = gastrointestinal transit time
References
1. Parkman HP, Orr WC. The gastrointestinal motility laboratory. Gastrointest Endosc Clin N Am. 2009. 19:171–184. viii
2. Merwid-Lad A, Trocha M, Ksiadzyna D, Sozanski T, Szelag A. Animals models for the gastrointestinal motility evaluation. Gastroenterol Pol. 2009. 16:201–206.
3. Lester NV, Roberts GD, Newell SM, Graham JP, Hartless CS. Assessment of barium impregnated polyethylene spheres (BIPS) as a measure of solid-phase gastric emptying in normal dogs--comparison to scintigraphy. Vet Radiol Ultrasound. 1999. 40:465–471.
4. Weber M, Stambouli F, Martin L, Dumon H, Biourge V, Nguyen P. Gastrointestinal transit of solid radiopaque markers in large and giant breed growing dogs. J Anim Physiol Anim Nutr (Berl). 2001. 85:242–250.
5. Chandler ML, Guilford G, Lawoko CR. Radiopaque markers to evaluate gastric emptying and small intestinal transit time in healthy cats. J Vet Intern Med. 1997. 11:361–364.
6. Haruta S, Kawai K, Jinnouchi S, Ogawara KI, Higaki K, Tamura S, et al. Evaluation of absorption kinetics of orally administered theophylline in rats based on gastrointestinal transit monitoring by gamma scintigraphy. J Pharm Sci. 2001. 90:464–473.
7. Marona HR, Lucchesi MB. Protocol to refine intestinal motility test in mice. Lab Anim. 2004. 38:257–260.
8. Sparkes AH, Papasouliotis K, Barr FJ, Gruffydd-Jones TJ. Reference ranges for gastrointestinal transit of barium-impregnated polyethylene spheres in healthy cats. J Small Anim Pract. 1997. 38:340–343.
9. Campbell JL, Williams CV, Eisemann JH. Characterizing gastrointestinal transit time in four lemur species using barium-impregnated polyethylene spheres (BIPS). Am J Primatol. 2004. 64:309–321.
10. Hinton JM, Lennard-Jones JE, Young AC. A ne method for studying gut transit times using radioopaque markers. Gut. 1969. 10:842–847.
11. Mittelstadt SW, Hemenway CL, Spruell RD. Effects of fasting on evaluation of gastrointestinal transit with charcoal meal. J Pharmacol Toxicol Methods. 2005. 52:154–158.
12. Tan-No K, Niijima F, Nakagawasai O, Sato T, Satoh S, Tadano T. Development of tolerance to the inhibitory effect of loperamide on gastrointestinal transit in mice. Eur J Pharm Sci. 2003. 20:357–363.
13. Ogata N, Ataka K, Morino H, Shibata T. Effect of wood creosote and loperamide on propulsive motility of mouse colon and small intestine. Pharmacology. 1999. 59:212–220.
14. Torjman MC, Joseph JI, Munsick C, Morishita M, Grunwald Z. Effects of isoflurane on gastrointestinal motility after brief exposure in rats. Int J Pharm. 2005. 294:65–71.
15. Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007. 318:426–430.
16. BioForum. Available at: http://www.protocol-online.org/forums/topic/7182-mouse-liver-anatomy/.
17. Saphier S, Rosner A, Brandeis R, Karton Y. Gastro intestinal tracking and gastric emptying of solid dosage forms in rats using X-ray imaging. Int J Pharm. 2010. 388:190–195.
18. Rao SS, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011. 23:8–23.
19. Parrish CR. Opioid analgesics and the gastrointestinal tract. Pract Gastroenterol. 2008. 64:37–50.
20. Szczesny G, Veihelmann A, Massberg S, Nolte D, Messmer K. Long-term anaesthesia using inhalatory isoflurane in different strains of mice-the haemodynamic effects. Lab Anim. 2004. 38:64–69.
21. Hildebrandt IJ, Su H, Weber WA. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J. 2008. 49:17–26.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download