Abstract
Objective
To investigate the safety, efficacy and long-term patency of parallel shunts (PS) in the management of the transjugular intrahepatic portosystemic shunt (TIPS) dysfunction.
Materials and Methods
Between March 2007 and October 2010, 18 patients (13 men and 5 women) who underwent TIPS revision with the creation of PS were evaluated retrospectively. In the first 10 patients, a 10-mm-diameter Wallgraft endoprosthesis was deployed; in the latter 8 patients, an 8-mm-diameter Fluency endoprosthesis was deployed.
Results
The creation of PS was technically successful in all patients. The mean ± standard deviation portosystemic pressure gradient before and after the procedure was 25.5 ± 7.3 mm Hg (range, 16-37 mm Hg) and 10.9 ± 2.3 mm Hg (range, 7-16 mm Hg), respectively. The duration of follow-up was 16.7 ± 10.8 months (range, 6-42 months). The primary shunt patency rates at 12 months after the creation of PS was 70% with Wallgraft endoprostheses and 87.5% with Fluency endoprostheses.
Since the transjugular intrahepatic portosystemic shunt (TIPS) was first introduced clinically in 1989, it has been considered an effective and safe procedure for treating complications of portal hypertension (1, 2). However, the main drawback of this procedure is the high rate of shunt dysfunction. The one-year primary patency rate after de novo TIPS creation is below 50%, and the long-term patency is disappointing (3, 4). TIPS dysfunction is usually associated with acute thrombosis within the stent, pseudointimal hyperplasia in the TIPS parenchymal tract or intimal hyperplasia in the outflow hepatic vein (5-7).
Recently, the use of expanded polytetrafluoroethylene (ePTFE)-covered stent grafts have shown remarkably improved long-term shunt patency by avoiding pseudointimal hyperplasia (8-10). Several methods have been utilized in treating the TIPS dysfunctions, including balloon angioplasty, stent insertion and the creation of a parallel shunt (PS). PS is generally used as the last therapeutic option when the initial shunt cannot be accessed by the guidewire or a puncture needle. However, there is little data on the clinical use of PS for the management of TIPS dysfunctions. The aim of this retrospective study was to investigate the safety, efficacy and long-term patency of PS.
This retrospective study was approved by the ethics committees of West China Hospital, Sichuan University. Between March 2007 and October 2010, a total of 132 patients were admitted to our institution for TIPS dysfunction, and 18 of these patients underwent the creation of PS because the original shunt could not be accessed. There were 13 male and five female patients, with a mean age of 50.2 ± 12.1 years (range, 31-69 years). The underlying liver diseases in the studied patients were hepatitis B cirrhosis (n = 12), alcoholic cirrhosis (n = 3), hepatitis C cirrhosis (n = 2) and Budd-Chiari syndrome (n = 1). Four patients had the Child-Pugh class A cirrhosis, nine had the class B disease, and five had the class C disease. Indications for initial TIPS creation were recurrent variceal bleeding in 15 patients and refractory ascites in three patients. The mean primary patency time was 12.7 ± 13.6 months (range, 1-53 months). The symptoms of TIPS dysfunction were recurrent variceal bleeding in 11 patients, recurrent ascites in two patients and abnormal ultrasonography (US) findings in five patients.
The procedure was explained in detail to all patients, and written consent was obtained from each patient before the procedure. All procedures were performed or supervised by one experienced interventional radiologist. PS was created using the classic TIPS technique (11, 12). In our series, the portal vein was punctured from the middle or right hepatic vein in 16 patients and from the inferior vena cava (IVC) in 2 patients. Once the catheter position in the portal vein was confirmed, a 5 Fr pigtail catheter (Cordis, Roden, the Netherlands) was introduced into the portal region, and a portography was obtained to outline the portal venous anatomy. Then, the liver parenchymal tract was dilated with an 8 × 60 mm angioplasty balloon (Cordis, Roden, the Netherlands). The stent-graft was deployed to cover the entire length of the shunt to the junction of the hepatic vein and IVC. In the first 10 patients, a 10-mm-diameter Wallgraft endoprosthesis (Boston Scientific, Galway, Ireland) was used. In the latter 8 patients, an 8-mm-diameter Fluency endoprosthesis (Bard, Karlsruhe, Germany) was used. After this, a final shunt venogram was performed. In addition, the portosystemic pressure gradient (PPG) was measured before and after the procedure. Patients with a PPG higher than 12 mm Hg underwent prophylactic embolization of varices with metal coils.
All patients received a single prophylactic dose of a second-generation cephalosporin one hour before the procedure. Intravenous heparin (3000 U) was administered immediately after a successful PS creation, except in the patients who had a coagulation disorder. Subsequently, the antiplatelet therapy with aspirin was maintained for life.
Technical success was defined as a correct positioning and deployment of the stent-graft parallel to the initial shunt. All patients were monitored for the shunt patency according to the follow-up schedule by the same medical team in the gastroenterology clinic. In detail, US was performed before the discharge and at 1, 3 and 6 months after the procedure and every 6 months thereafter or whenever was clinically necessary. TIPS dysfunction was suspected on US if the intrastent flow velocity was less than 60 cm/s or higher than 120 cm/s or if there was a change in the direction of the flow in the intrahepatic portal branches in comparison with the previous US findings. TIPS dysfunction was defined as the shunt narrowing more than 50%, a PPG higher than 12 mm Hg or both. Primary patency was defined as the length of time without reintervention.
The results are expressed as the mean ± standard deviation. The primary patency rates at 12 months were compared between the Wallgraft and the Fluency patients with the log-rank test. A p value of less than 0.05 was considered to indicate a statistically significant difference. All calculations were performed by using the SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA) for the Windows.
Technical success of PS creation with a covered stent-graft was achieved in all 18 patients (100%). No severe complications occurred during any procedure. The 30-day mortality rate was 0%. The mean PPG decreased from 25.5 ± 7.3 mm Hg (range, 16-37 mm Hg) before to 10.9 ± 2.3 mm Hg (range, 7-16 mm Hg) after PS creation. In one patient (#6), it was necessary to deploy two Wallgraft endoprostheses to cover the parenchymal tract and the hepatic vein. In the other 17 patients, a single device was used, with a stent-graft length of 6 cm in 13 patients and 8 cm in four patients. In four patients (#2, #3, #9, #10) with Wallgraft endoprostheses, the PPG was not reduced to below 12 mm Hg; therefore, a 10 × 40 angioplasty balloon was utilized to dilate the stent-graft to its nominal diameter. Four patients (#2, #3, #16, #17) with a PPG higher than 12 mm Hg received the prophylactic embolization of varices with metal coils. Patient characteristics, technical data and outcomes are summarized in Table 1.
After a mean follow-up period of 16.7 ± 10.8 months (range, 6-42 months), four patients (22.2%) had died. Two patients (#1, #7) died of liver failure, one patient (#9) died of hepatorenal syndrome, and one patient (#14) died of an unknown cause. Two patients (#2, #13) were lost to follow-up at 6 and 18 months after the procedure, respectively, but had no signs of shunt dysfunction at the last evaluation. Hepatic encephalopathy developed in three patients (#1, #8, #18) after PS creation, and it resolved after protein restriction and lactulose administration.
Four patients developed recurrent TIPS dysfunction during the follow-up period, of whom three (#4, #5, #12) presented with recurrent gastrointestinal bleeding and one (#13) was diagnosed with US findings. One patient (#4) received a third PS creation, because the initial two shunts could not be accessed (Fig. 1). Angioplasty of the second PS and insertion of an additional stent-graft was performed in the other three patients (Fig. 2). The primary shunt patency rate at 12 months after PS creation was 70% with the Wallgraft endoprostheses and 87.5% with the Fluency endoprostheses (log-rank test, p = 0.358).
Transjugular intrahepatic portosystemic shunt is widely accepted as an effective procedure in treating complications of portal hypertension (13-16). However, the application of this intervention has been limited due to a high rate of TIPS dysfunction. Therefore, a close shunt surveillance and frequent secondary interventions are the necessary follow-up requirements to maintain the shunt efficiency.
Transjugular intrahepatic portosystemic shunt dysfunction has been attributed to three different mechanisms: acute thrombosis within the stent; pseudointimal hyperplasia secondary to the biliary leaks of the lacerated bile ducts into the shunt lumen; and intimal hyperplasia in the outflow hepatic vein (7, 17). Additionally, preliminary stent-graft position within the outflow hepatic vein plays an important role in the TIPS patency. The turbulence and shear stress from the increased shunt flow can provoke the acceleration of pseudointimal hyperplasia and predispose the patients to shunt dysfunction. Clark and his colleagues have demonstrated that TIPS extending to the hepatocaval junction has a better patency than the shunts terminating in the hepatic vein (18). Based on the previous clinical studies and our experience, we were careful in bridging the complete tract to the IVC in the present series.
To date, several interventions have been utilized to treat the dysfunctional TIPS, including angioplasty, placement of an additional stent-graft and PS creation (19). The preferred intervention is a placement of an additional stent after negotiating the initial stenotic or the occluded shunt. This not only allows for the restoration of the TIPS functions, but it may also be related to the improved shunt patency aided by the reinforcement and the radial strength of the second stent. If access to the existent shunt appears to be impossible, the creation of PS may be necessary, although in some instances the catheterization of a different hepatic vein may be difficult. In 1998, Dabos et al. (19) first described a series of 29 patients undergoing the PS insertion, suggesting that the PS patency without further intervention is slightly superior to the patency of the first shunts. In 2006, Helmy et al. (20) described the natural history of PS in 40 patients with the TIPS insufficiency. After a mean follow-up period of 11.6 months, both the PS and the index shunt in the non-PS group behaved in a similar way regarding the cumulative primary shunt patency rates at 6, 12, 24 and 36 months (60.3%, 33.6%, 22.4% and 7% vs. 65.1%, 46.1%, 18.5% and 8.5%).
Compared with the previously published reports, our series had a relatively small number of patients. The application of the Doppler ultrasonography and the contrast-enhanced ultrasound in detecting shunt dysfunction is widely accepted, which may identify stenosis in the early stages. In our institution, when the primary guidewire recanalization via the right jugular vein fails, a direct puncture of the end of the stent or revision via the left jugular vein may be attempted. Moreover, some investigators have described a combined transjugular and transhepatic approach. After puncturing the stent lumen transhepatically, a guidewire is passed through the shunt and snared through the internal jugular access (21, 22). Considering the improvement of surveillance methods and the multiple interventional techniques used for TIPS revision, the number of patients undergoing the PS creation has decreased gradually.
In our series, the transcaval PS creation was carried out in one patient with Budd-Chiari syndrome and in another patient whose hepatic vein could not be catheterized due to the previous shunt blockage. Transcaval TIPS was first described by Haskal et al. (23) in 1996. In their cases of the study, the hepatic veins were inaccessible or inadequate as the cephalad locations of the portal vein bifurcation were close to the hepatic veins.
In addition, various experimental and clinical studies have verified the application of ePTFE-covered stent-grafts in de novo TIPS creation and TIPS revision (5, 8, 21, 24, 25). The ePTFE is utilized as a cover material for the stent-grafts, separating the blood flow within the shunt from the liver parenchyma and from the injured outflow hepatic vein. After the creation of TIPS with an ePTFE-covered stent-graft, the shunt flow is maintained by inhibiting the overgrowth of the pseudointimal hyperplasia in the parenchymal tract or along the outflow hepatic vein. Park JS also found that the intraluminal irradiation with Holmium-166 for the TIPS significantly improved the TIPS patency in a swine model (26). In the present study, a PS creation with the ePTFE-covered stent-grafts was performed in the latter nine patients. The differences between the Wallgraft endoprostheses and the Fluency endoprostheses did not reach statistical significance due to the small number of patients enrolled, but our data do show a tendency favoring the ePTFE-covered stent grafts.
The limitations of this study are multiple: the small patient series (n = 18); the lack of a contemporaneous control group; a mean follow up of only 16.7 ± 10.8 months; and the retrospective design. In addition, only the clinical outcomes were studied without the performance of the direct shunt venograms during the follow-up to evaluate the PS patency. Despite these limitations, our data suggest that the creation of PS with the covered stent-grafts is safe, feasible and clinically effective in the treatment of TIPS dysfunction, providing a favorable primary shunt patency. However, these observations need to be further validated.
Figures and Tables
Fig. 1
69-year-old man following TIPS creation with Wallgraft endoprosthesis presented with recurrent variceal bleeding.
A. Direct portogram showed occluded initial stent and numerous collaterals veins. B. After PS creation with second Wallgraft endoprosthesis, portogram showed good flow through shunt, without opacification of collateral veins. C. PS dysfunction developed 13 months later, and third PS was created with Wallgraft endoprosthesis. D. Final portogram showed widely patent shunt. TIPS = transjugular intrahepatic portosystemic shunt, PS = parallel shunt
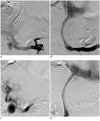
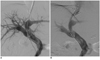
Fig. 2
40-year-old man with alcoholic liver cirrhosis and recurrent variceal bleeding.
A. Initial portogram showed occluded intrahepatic portosystemic shunt. B. Final portogram after revision with PS creation showed good flow through shunt. This PS was created with Fluency endoprosthesis. PS = parallel shunt
References
1. Boyer TD, Haskal ZJ. American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010. 51:306.
2. Richter GM, Palmaz JC, Nöldge G, Rössle M, Siegerstetter V, Franke M, et al. [The transjugular intrahepatic portosystemic stent-shunt. A new nonsurgical percutaneous method]. Radiologe. 1989. 29:406–441.
3. Haskal ZJ, Pentecost MJ, Soulen MC, Shlansky-Goldberg RD, Baum RA, Cope C. Transjugular intrahepatic portosystemic shunt stenosis and revision: early and midterm results. AJR Am J Roentgenol. 1994. 163:439–444.
4. Saxon RS, Ross PL, Mendel-Hartvig J, Barton RE, Benner K, Flora K, et al. Transjugular intrahepatic portosystemic shunt patency and the importance of stenosis location in the development of recurrent symptoms. Radiology. 1998. 207:683–693.
5. Nishimine K, Saxon RR, Kichikawa K, Mendel-Hartvig J, Timmermans HA, Shim HJ, et al. Improved transjugular intrahepatic portosystemic shunt patency with PTFE-covered stent-grafts: experimental results in swine. Radiology. 1995. 196:341–347.
6. Tanihata H, Saxon RR, Kubota Y, Pavcnik D, Uchida BT, Rosch J, et al. Transjugular intrahepatic portosystemic shunt with silicone-covered Wallstents: results in a swine model. Radiology. 1997. 205:181–184.
7. Cura M, Cura A, Suri R, El-Merhi F, Lopera J, Kroma G. Causes of TIPS dysfunction. AJR Am J Roentgenol. 2008. 191:1751–1757.
8. Rossi P, Salvatori FM, Fanelli F, Bezzi M, Rossi M, Marcelli G, et al. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology. 2004. 231:820–830.
9. Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004. 126:469–475.
10. Cejna M, Peck-Radosavljevic M, Thurnher SA, Hittmair K, Schoder M, Lammer J. Creation of transjugular intrahepatic portosystemic shunts with stent-grafts: initial experiences with a polytetrafluoroethylene-covered nitinol endoprosthesis. Radiology. 2001. 221:437–446.
11. Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994. 330:165–171.
12. Ochs A, Rössle M, Haag K, Hauenstein KH, Deibert P, Siegerstetter V, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for refractory ascites. N Engl J Med. 1995. 332:1192–1197.
13. Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010. 59:988–1000.
14. García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010. 362:2370–2379.
15. Feldstein VA, Patel MD, LaBerge JM. Transjugular intrahepatic portosystemic shunts: accuracy of Doppler US in determination of patency and detection of stenoses. Radiology. 1996. 201:141–147.
16. Tzeng WS, Wu RH, Lin CY, Chen JJ, Sheu MJ, Koay LB, et al. Prediction of mortality after emergent transjugular intrahepatic portosystemic shunt placement: use of APACHE II, Child-Pugh and MELD scores in Asian patients with refractory variceal hemorrhage. Korean J Radiol. 2009. 10:481–489.
17. Siegerstetter V, Huber M, Ochs A, Blum HE, Rössle M. Platelet aggregation and platelet-derived growth factor inhibition for prevention of insufficiency of the transjugular intrahepatic portosystemic shunt: a randomized study comparing trapidil plus ticlopidine with heparin treatment. Hepatology. 1999. 29:33–38.
18. Clark TW, Agarwal R, Haskal ZJ, Stavropoulos SW. The effect of initial shunt outflow position on patency of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2004. 15(2 Pt 1):147–152.
19. Dabos KJ, Stanley AJ, Redhead DN, Jalan R, Hayes PC. Efficacy of balloon angioplasty, restenting, and parallel shunt insertion for shunt insufficiency after transjugular intrahepatic portosystemic stent-shunt (TIPSS). Minim Invasive Ther Allied Technol. 1998. 7:287–293.
20. Helmy A, Redhead DN, Stanley AJ, Hayes PC. The natural history of parallel transjugular intrahepatic portosystemic stent shunts using uncovered stent: the role of host-related factors. Liver Int. 2006. 26:572–578.
21. Echenagusia M, Rodriguez-Rosales G, Simo G, Camuñez F, Bañares R, Echenagusia A. Expanded PTFE-covered stent-grafts in the treatment of transjugular intrahepatic portosystemic shunt (TIPS) stenoses and occlusions. Abdom Imaging. 2005. 30:750–754.
22. Chan CY, Liang PC. Recanalization of an occluded intrahepatic portosystemic covered stent via the percutaneous transhepatic approach. Korean J Radiol. 2010. 11:469–471.
23. Haskal ZJ, Duszak R Jr, Furth EE. Transjugular intrahepatic transcaval portosystemic shunt: the gun-sight approach. J Vasc Interv Radiol. 1996. 7:139–142.
24. Haskal ZJ. Improved patency of transjugular intrahepatic portosystemic shunts in humans: creation and revision with PTFE stent-grafts. Radiology. 1999. 213:759–766.
25. Cejna M, Peck-Radosavljevic M, Thurnher S, Schoder M, Rand T, Angermayr B, et al. ePTFE-covered stent-grafts for revision of obstructed transjugular intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol. 2002. 25:365–372.
26. Park JS, Oh JH, Kim DY, Park YK, Park SJ, Kim SJ. Effects of intraluminal irradiation with Holmium-166 for TIPS stenosis: experimental study in a swine model. Korean J Radiol. 2007. 8:127–135.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download