Abstract
We describe a unique case of a patient who presented with a linear, transverse, and incidentally-detected main pancreatic duct dilatation that was caused by the intrapancreatic-replaced common hepatic artery, detected on the MDCT, MRCP and endoscopic retrograde cholangiopancreatography. We believe this case to be the first of its kind reported in the literature.
Rarely the common hepatic artery (CHA) crosses the intrapancreatic parenchyma at the pancreas head or neck, making it problematic to consider pancreatic head resection (1). In addition, organs such as esophagus, duodenum, common bile duct, or ureters can be compressed by blood vessels, causing symptoms like dysphagia, jaundice, or hydronephrosis. As far as we know, dilatation of main pancreatic duct by intrapancreatic-replaced CHA has been not reported on the literature. We describe a unique case of intrapancreatic CHA causing main pancreatic duct dilatation.
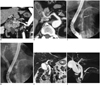
A 63-year-old male was admitted to the hospital for an elevation of hepatic enzymes and dark colored urine. He had experienced fatigue and malaise. He had no abdominal pain and neither significant medical history nor family history. Based on the viral maker assay, he was diagnosed with acute hepatitis and possible hepatitis A infection. On routine abdominal ultrasonography to rule out other organic causes, main pancreatic duct dilatation was incidentally found. The patient underwent contrast enhancement liver dynamic CT and MRCP for further characterization of duct dilatation and evaluation of liver. Contrast enhancement CT scan was taken by bolus tracking method. Liver dynamic CT consisting of four phase (unenhanced, arterial [15 seconds], portal [40 seconds] and delayed [3 minutes] scan) images were acquired with an 16-channel multi-detector raw CT (Mx8000 IDT 16 CT scanner; Philips Medical System, Best, The Netherlands). In addition, MRCP images were acquired with a 3.0 Tesla MR scanner (Achieva 3.0T X-series, Philips Medical System, Best, The Netherlands). On axial and coronal curved multiplanar reconstruction images using arterial phase scan, the CHA was arising from the superior mesenteric artery (SMA) and traveling through the pancreatic head parenchyma and across the main pancreatic duct, supero-posteriorly (Fig. 1A, B). The upstream main pancreatic duct crossing the replaced CHA shows diffuse tubular dilatation without abnormal wall thickening or enhancement. Endoscopic retrograde cholangiopancreatography (ERCP) shows abrupt transverse filling defect at pancreas head and stasis of the contrast medium during the ERCP procedure (Fig. 1C, D). Routine MRCP images revealed a compression of main pancreatic duct by the replaced CHA seen as a tubular signal void appearing as dark signal intensity at the corresponding area of the ERCP, which is flow void on the CHA, on both coronal T2 weighted image (repetition time [TR]/echo time [TE], 1728/80; section thickness 3 mm) and maximum intensity projection of respiratory triggered 3-dimensional turbo spin-echo MRCP images (TR/TE, 1868/600) (Fig. 1E, F). The main pancreatic duct was compressed and dilated by the intrapancreatic-replaced CHA, resulted in prolonged stasis of the contrast medium in the upstream pancreatic duct noted on ERCP. However, this patient was asymptomatic and gave no evidence of history suggesting acute pancreatitis. This finding posed a dilemma to the clinician and surgeon because the prolonged stasis of contrast medium in the dilated pancreatic duct is a potential cause of acute pancreatitis. However, the clinician and surgeon decided upon close observation of patient status instead of the distal pancreatectomy because the high morbidity and mortality of surgical treatment was not beneficial to an asymptomatic patient.
According to the literature review, the proper hepatic artery originating from the SMA occurs in 0.9 to 4.5% of individuals (2). The replaced CHA courses along the portocaval space, supero-posterior margin of the pancreas, and enter the hepatoduodenal ligament. The majority of these replaced CHA are located at the peripancreatic retroperitoneal space. Only a case of intrapancreatic CHA was reported (1).
The prevalence of the intrapancreatic-replaced CHA is not well known. The intrapancreatic-replaced CHA is an incidental finding and usually asymptomatic and no problematic. However, surgeons are challenged by the intrapancreatic-replaced CHA or replaced right hepatic artery, especially in considering the pancreatic head resection. Preservation of the intrapancreatic-replaced CHA is important and dissection from imbedded pancreatic parenchyma is sometimes difficult (1, 3).
Common bile duct and ureters tend to be compressed by adjacent or aberrant origin arteries. Compression of the gastrointestinal tract commonly occurs at the esophagus and duodenum. The esophagus is compressed by the aberrant right subclavian artery, causing dysphagia. The development of the aberrant right subclavian artery is related to the degeneration of the entire right fourth aortic arch during the embryogenesis (4, 5). The third portion of the duodenums is mechanical compression between the SMA and abdominal aorta known as the SMA syndrome. SMA syndrome occurs mainly through loss of mesenteric and retroperitoneal fat representing as a low aorto-mesenteric angle but anatomic factors like a short ligament of Treitz or low origin of the SMA, trauma, dietary disorders, postoperative state are also considered predisposing conditions (6, 7).
A unique case report of common bile duct obstruction by the postero-superior pancreatico-duodenal artery is similar to our case report (8). Common bile duct obstruction by arterial compression results in bile stasis and is prone to form biliary stones. In addition, ureter obstruction by vascular lesion is relatively common and either artery or vein can be the cause. Abdominal aortic aneurysm, iliac artery aneurysm, accessory renal arteries and other aberrant arteries are arterial causes of ureter obstruction. Normal venous structures such as accessory renal veins, iliac vein or gonadal veins can cause ureter compression and circumcaval ureter is a congenital anomaly causing ureter obstruction (9, 10).
However, as far as we know, this is the first case report of compression of the main pancreatic duct by intrapancreatic-replaced CHA. Although prolonged stasis of the contrast medium was noted on ERCP, the patient did not have any symptoms or signs of pancreatitis. So the pancreatic duct compression by the intrapancreatic-replaced CHA was an incidental finding but we pay attention to the findings of main pancreatic duct dilatation and stasis of the contrast medium that can predispose to acute pancreatitis. In summary, intrapancreatic-replaced CHA is a very rare anatomic anomaly and a cause of pancreatic duct dilatation by aberrant course and compression of replaced CHA.
Figures and Tables
Fig. 1
Imaging findings of main pancreatic duct compression by intrapancreatic-replaced common hepatic artery in 63-year-old man.
A, B. On curved coronal (A) and axial (B) multiplanar reconstruction images obtained on arterial phase (15 seconds after contrast injection on bolus tracking method), common hepatic artery (black arrow) arises from superior mesenteric artery (white arrow) is crossing pancreatic parenchyma and causing compression of main pancreatic duct (small double arrows). Celiac trunk is normally arising from aorta, superiorly (A, *). C, D. Endoscopic retrograde cholangiopancreatography shows tubular filling defect of main pancreatic duct (C, arrow) and dilatation of upstream main pancreatic duct in addition to stasis of contrast medium (D). E. Coronal T2-weighted image (repetition time [TR]/echo time [TE], 1728/80; slice thickness, 3 mm) shows intrapancreatic-replaced common hepatic artery representing as tubular signal void (arrow), which is crossing and compressing main pancreatic duct (arrowheads). F. Coronal maximum-intensity projection of respiratory triggered 3-dimensional turbo spin echo MRCP image (TR/TE, 1868/600) demonstrates clear defect of main pancreatic duct at neck area (arrow) and dilatation of upstream main pancreatic duct.
References
1. Furukawa H, Shimada K, Iwata R, Moriyama N. A replaced common hepatic artery running through the pancreatic parenchyma. Surgery. 2000. 127:711–712.
2. Abdullah SS, Mabrut JY, Garbit V, De La Roche E, Olagne E, Rode A, et al. Anatomical variations of the hepatic artery: study of 932 cases in liver transplantation. Surg Radiol Anat. 2006. 28:468–473.
3. Jah A, Jamieson N, Huguet E, Praseedom R. The implications of the presence of an aberrant right hepatic artery in patients undergoing a pancreaticoduodenectomy. Surg Today. 2009. 39:669–674.
4. Bisognano JD, Young B, Brown JM, Gill EA, Fang FC, Zisman LS. Diverse presentation of aberrant origin of the right subclavian artery: two case reports. Chest. 1997. 112:1693–1697.
5. Alper F, Akgun M, Kantarci M, Eroglu A, Ceyhan E, Onbas O, et al. Demonstration of vascular abnormalities compressing esophagus by MDCT: special focus on dysphagia lusoria. Eur J Radiol. 2006. 59:82–87.
6. Welsch T, Büchler MW, Kienle P. Recalling superior mesenteric artery syndrome. Dig Surg. 2007. 24:149–156.
7. Raman SP, Neyman EG, Horton KM, Eckhauser FE, Fishman EK. Superior mesenteric artery syndrome: spectrum of CT findings with multiplanar reconstructions and 3-D imaging. Abdom Imaging. 2012. 37:1079–1088.
8. Watanabe H, Iwase H, Sugitani M, Watanabe T. Compression of the common bile duct by the posterosuperior pancreaticoduodenal artery: case report. Abdom Imaging. 2005. 30:214–217.
9. Cole PE, Saluja S. Pollack HM, McClennan BL, Dyer RB, editors. Vascular Abnormalities of the Lower Urinary Tract. Clinical Urography. 2000. 2nd ed. Boston: W.B Saunders;2563–2580.
10. Stern JM, Park S, Anderson JK, Landman J, Pearle M, Cadeddu JA. Functional assessment of crossing vessels as etiology of ureteropelvic junction obstruction. Urology. 2007. 69:1022–1024.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download