Abstract
Objective
To investigate CT and 18F-flurodeoxyglucose (18F-FDG) positron-emission tomography/CT findings of primary endobronchial marginal zone B-cell lymphoma of the bronchus-associated lymphoid tissue (BALT).
Materials and Methods
From June 2006 through April 2012, seven patients (six female, one male; age range, 21-61 years; mean age, 49 years) were examined who were pathologically diagnosed with the primary endobronchial marginal zone B-cell lymphoma of BALT. We evaluated the locations and characteristics of the lesions on CT and 18F-FDG-PET/CT scans. The lesions were classified into the following three patterns: 1) solitary intraluminal nodule; 2) several tiny nodular protrusions; and 3) diffuse wall thickening.
Results
A solitary intraluminal nodule was observed in four patients (57.1%), several tiny nodular protrusion in two patients (28.6%), and diffuse wall thickening in one patient (14.3%). The lesions were categorized into 3 major locations: confined to the trachea (n = 3), confined to the lobar bronchus (n = 2), and diffuse involvement of the trachea and both main bronchi (n = 2). All lesions demonstrated homogeneous iso-attenuation as compared with muscle on pre- and post-enhancement scans. Secondary findings in the lungs (n = 3; 42.9%) included postobstructive lobar atelectasis (n = 1), air trapping (n = 1), and pneumonia (n = 1). On 18F-FDG-PET/CT (n = 5), 4 lesions showed homogeneous uptake with maximum standardized uptake values (mSUV), ranging 2.3-5.7 (mean mSUV: 3.3). One lesion showed little FDG uptake.
Mucosa-associated lymphoid tissue refers to the lymphoid tissue that is located under the epithelia of the gastrointestinal, respiratory, and urogenital tracts and their anlages. Mucosa-associated lymphoid tissue that is located under the respiratory tract epithelia is called bronchus-associated lymphoid tissue (BALT).
Marginal zone B-cell lymphoma of BALT, which originates in the marginal zone and invades the bronchial epithelial tissue, is histopathologically characterized by cellular infiltrations of lymphoepithelial cells with a predominance of smaller cell types. BALT lymphomas have been associated with Sjögren's syndrome, dysgammaglobulinemia, amyloid deposits, collagen vascular diseases, Helicobacter pylori infection, and AIDS (1, 2).
The most common findings on the computed tomography (CT) of the marginal zone B-cell lymphoma of BALT were initially reported as parenchymal consolidation with air bronchograms, caused by the cellular lymphocytic infiltrates that expanded into the interstitium and compressed the adjacent alveoli (3).
Positron-emission tomography (PET) with 18F-flurodeoxyglucose (18F-FDG) is used to evaluate glucose metabolism in lung lesions. Because marginal zone B-cell lymphomas of BALT show indolent growth, it is expected that these tumors will demonstrate little FDG uptake. Marginal zone B-cell lymphomas of BALT shows indolent growth, remains localized for a long time, and responds well to local therapy (3). The 5-year survival rate is > 80% after complete resection (4, 5). For this reason, it is thought to be important to understand the radiologic spectrum of endobronchial marginal zone B-cell lymphoma of BALT for differentiating it from other endobronchial tumors. However, to the best of our knowledge, there are few case reports which report on the short cases of primary endobronchial marginal zone B-cell lymphoma of BALT, and only a limited number of studies has used 18F-FDG-PET/CT to study lymphoma (6-11). Thus, the purpose of this study is to present CT and 18F-FDG-PET/CT findings of primary endobronchial marginal zone B-Cell lymphoma of BALT.
This retrospective study was approved by the institutional review board of the Asan Medical Center. The pathologic reports were reviewed to identify the patients with endobronchial marginal zone B-cell lymphoma of BALT, who were examined at the hospital between June 2006 and April 2012. A total of 7 patients who met all of the following criteria were selected: 1) histologic features of Marginal zone B-cell lymphoma of BALT involving airway; and 2) no evidence of lymphoma involvement at the other organs at the time of diagnosis. The lesions that invade the airway as a consequence of either metastasis or direct extension were excluded. Clinical records were available for all patients and were reviewed to determine the presenting clinical symptoms, laboratory data, and bronchoscopic and pathological findings. Patient age, sex, clinical symptoms, and laboratory data are listed in Table 1.
All patients claimed to have no history of smoking. Systemic screening did not reveal involvement with any other organs, and all of the tumors were considered primary endobronchial tumors. Five patients underwent 18F-FDG-PET/CT.
Computed tomography images were available for all patients. Chest CT scans with contrast enhancement were performed on all patients using 16-channel MDCT (Somatom Sensation 16; Siemens medical systems, Forchheim, Germany and LightSpeed Ultra 16; GE Healthcare, Milwaukee, WI, USA). The CT parameters were as follows: 0.75-mm collimation; 5 mm slice thickness; 5 mm intervals; reconstruction with standard algorithm; 120-140 kVp and 180 mAs.
Computed tomography images were reviewed by two experienced chest radiologists. All chest CT images were reviewed by two chest radiologists, and all decisions were reached by consensus. The CT images were evaluated to determine the location, size, thickness, shape, characteristics, enhancement or homogeneity of the tracheobronchial mural lesion, lymphadenopathy, and secondary or combined findings in the lungs.
In the 5 patients (patients 1, 3, 4, 6 and 7) who underwent 18F-FDG-PET/CT in order to examine their lesions in the tracheobronchial tree, we determined the maximum standardized uptake values (mSUV) of each nodule and the level of involvement with any other organs (Table 2).
Based on the predominant radiologic abnormalities on the study, the primary endobronchial marginal zone B-cell lymphomas of BALT were categorized into 3 major patterns: 1) solitary intraluminal nodule; 2) several tiny nodular protrusions; and 3) diffuse wall thickening.
All patients underwent bronchoscopy and bronchoscopic biopsy. Correlations between the radiologic and bronchoscopic findings were examined by two radiologists and one expert interventional bronchoscopist, and the decisions were reached by consensus. Correlations between the radiologic and pathological findings were examined by two radiologists and a surgical pathologist.
Pathological diagnoses of primary endobronchial marginal zone B-cell lymphoma of BALT were made using the specimens obtained from the bronchoscopic biopsies. The biopsy specimens were available from all patients and reviewed by the surgical pathologist. All patients underwent six immunohistochemical staining for CD3, CD5, CD10, CD20, BCL6, and KI-67 (termed the BALT-battery at the authors' hospital), using the bronchoscopic biopsy specimens.
As shown in Table 1, the clinical findings and outcomes after the treatment were identified. Seven patients were young or middle-aged women, and one patient was a man. The survival rate was 100% with a mean of 23.9 months (range: 1-48 months) after the radiotherapy (patients 3, 6 and 7), chemotherapy (patients 1 and 2), cryotherapy (patient 4), or no treatment (patient 5). The clinical outcomes included complete remission (patients 3, 4, and 6) and partial remission (patients 1 and 2) on the follow-up CT and bronchoscopy.
As shown in Table 2, the radiologic and 18F-FDG-PET/CT findings were identified. Based on the radiologic features of the 7 patients, these findings were classified into 3 patterns: solitary intraluminal nodule, several tiny nodular protrusion, and diffuse wall thickening.
The solitary intraluminal nodule pattern (patients 3-6) was characterized by the presence of a single nodule (average diameter: 17.5 mm; range: 11-15mm) (Figs. 1, 2). All patients with the solitary nodular pattern were initially thought to have tracheal or bronchogenic carcinomas or minor salivary gland tumors, such as adenoid cystic or mucoepidermoid carcinoma.
The pattern of several tiny nodular protrusion pattern (patient 2 and 7) was radiologically characterized by multiple round-shaped nodular protrusions, all < 5 mm size without calcification (Fig. 3). Based on the morphology alone, these patients were initially thought to have endobronchial tuberculosis or tracheobronchopathia osteochondroplastica.
The pattern of diffuse wall thickening (patient 1) was radiologically characterized by diffuse and smooth tracheobronchial-wall thickening (Fig. 4). The patient was initially suspected of having acute tracheobronchial tuberculosis with exudative membrane.
Based on the location, the lymphomas in these patients were categorized into 3 major locations: confined trachea (patients 3, 4 and 7), confined lobar bronchus (patients 5 and 6), and diffuse involvement of the trachea, carina, and main bronchus (patients 1 and 2).
All lesions showed homogeneous iso-attenuation to muscle on pre- and post-enhancement.
The secondary findings in the lungs included 1 patient with postobstructive lobar atelectasis (patient 6), 1 patient with lobular air trapping (patient 5), and 1 patient with postobstructive mild bronchopneumonia in both lungs (patient 1). One patient improved after receiving antibiotics therapy (patient 1). This patient had enlarged lymph nodes at the right axillary and right interlobar level, with mSUV of 2.2 on 18F-FDG-PET/CT and possible reactive hyperplasia or lymphadenopathy. There was no evidence of lung or systemic involvement of the BALT lymphomas in any of these patients.
On 18F-FDG-PET/CT (n = 5), four lesions showed homogeneous uptake with maximum standardized uptake values (mSUV), ranging 2.3-5.7 (mean mSUV: 3.3) and one lesion showed no significant hypermetabolic activity (Table 2).
The radiologic finding of a 'solitary intraluminal nodule pattern' was characterized by a nonspecific intraluminal polypoid mass on bronchoscopy. The radiologic finding of a 'several tiny nodular protrusion pattern' was characterized by multiple, small, nodular mucosal lesions with cobblestone appearance on bronchoscopy. The radiologic finding of a 'diffuse wall thickening pattern' was characterized by well-defined diffuse wall thickening with exudates on bronchoscopy. All radiologic and bronchoscopic findings were nonspecific on the initial diagnosis.
Regardless of the classification of the CT abnormalities according to the three designated patterns, the corresponding pathologic samples had the same microscopic appearance, showing lymphoma cell infiltration. There was no destruction of the bronchial wall or tumor necrosis. Histological examination demonstrated diffuse infiltration of the small lymphoid cells that surround the reactive follicles, and there were few lymphoepithelial lesions. The BALT lymphomas were low-grade B-lymphocyte lymphomas, composed of small lymphocytes with focal plasmacytoid features. The small lymphoid cells were B cells, as identified by CD20 staining, that demonstrated negative staining by CD3, CD5, CD10, BCL6, and Ki-67. The histological diagnosis was consistent with the low-grade marginal zone B-cell lymphoma originating from BALT (Figs. 1, 3).
Bronchus-Associated Lymphoid Tissue is a lymphoid aggregate located in the submucosal area of the bronchioles and plays a central role in the mucosal immunity of the airways, by inducing the accumulation of secretory immunoglobulin A (IgA)-producing cells. Long-lasting antigen stimuli promote the hyperplasia of BALT, which may develop into pulmonary mucosa-associated lymphoma (12, 13). Chronic inflammatory diseases could evoke oligo- or monoclonal cell proliferation in the mucosal lymphoid tissue, resulting in the development of lymphoma (14). Our patients included 1 patient with rheumatoid arthritis and 2 patients with allergic rhinitis.
It is rare for an endobronchial lesion to be the primary presentation of lymphoma. Most cases of endobronchial lymphoma occur along with systemic diseases, more often in Hodgkin's lymphoma than non-Hodgkin's lymphoma (15-17). Primary marginal zone B-cell lymphoma of BALT in the central airway is extremely rare. Endobronchial lymphoma is classified into two patterns according to the pattern of involvement (6). Pattern 1 demonstrates diffuse submucosal infiltrates that originate from hematogenous or lymphangitic spread, in the presence of systemic lymphoma. Pattern 2 demonstrates airway involvement of a localized mass, due to the direct spread of lymphoma from the adjacent lymph nodes, or a lymphoma that arise de novo from BALT. Localized primary endobronchial lymphoma that is confined to the tracheobronchial tree without dissemination which may be classified as the Pattern 2 is extremely rare. All of our patients only presented with endobronchial lesions in the central airway, without mediastinal, hilar lymphadenopathy, or pulmonary parenchymal involvement of BALT. Moreover, none of our patients presented with systemic involvement according to the whole-body lymphoma work-up and 18F-FDG-PET/CT, suggesting de novo lymphoma in the central airway.
In the four patients in this series, the main CT features included a mural, polypoid, ovoid endobronchial mass of the trachea or lobar bronchus with postobstructive air trapping, atelectasis, or pneumonia. There are a few reports on endobronchial BALT lymphoma in the lobar bronchus with lobar atelectasis and left main bronchus with unilateral hyperlucent left lung, but most of these are the case report findings of a single patient (6, 18, 19).
In the three patients in this series, the main CT features included multiple, tiny, nodules or diffuse wall thickening along the trachea and main stem bronchi. There is one case report in the literature that describes diffuse narrowing of the tracheobronchial tree with endobronchial nodularities of extranodal marginal zone lymphoma, occurring along the trachea and central airway (20). However, to the best of our knowledge, BALT lymphoma manifesting as diffuse wall thickening in the central airway on CT has not been reported. As such, our novel observation of the primary endobronchial marginal zone B-cell lymphoma of BALT was that it can manifest in not only the small airways and lung parenchyma, but also in the larger such as the trachea and major bronchi. On bronchoscopy, there was a well-circumscribed nodule with a smooth margin which mimicked the submucosal lesion in each patient. This finding correlates well with the CT findings.
Based on the results of the study, the primary endobronchial marginal zone B-cell lymphoma of BALT can be classified into 3 patterns: solitary intraluminal nodule, several tiny nodular protrusion, and diffuse wall thickening. That is, primary endobronchial marginal zone B-cell lymphoma of BALT needs to be differentiated from other variable large-airway diseases, including neoplastic and nonneoplastic lesions, and the differential diagnosis of each patient is required, depending on the radiologic patterns of the endobronchial BALT lymphoma. Nevertheless, it is not easy to differentiate endobronchial BALT lymphoma from other airway diseases. As such, the use of the radiologic-bronchoscopic correlation with histological confirmation and 18F-FDG-PET/CT are necessary to confirm the diagnosis and to exclude any other origins of the primary lymphoma and systemic involvement.
According to several studies by Beal et al. (9) and Bae et al. (10), marginal zone B-cell lymphoma shows identifiable and demonstrable, but not avid, FDG uptake with mSUVs ranging in 2.6-26 (mean mSUV: 6.8) and mSUVs in the range of 2.2-6.3 (mean mSUV: 4.2 ± 1.48), respectively. In our series, the maximum FDG uptake on 18F-FDG-PET/CT was 3.3 (range: 2.3-5.7), similar to those mentioned in the earlier studies (9, 10). It is expected that the indolent growth and metabolic stability of these lesions cause the relatively low level of FPG uptake.
Treating BALT lymphoma is controversial, ranging from only observation to surgical resection alone, or in combination with chemotherapy or radiotherapy (18). However, BALT lymphomas tend to remain localized until late in the natural course. The histological progression from a low-grade BALT lymphoma to a high-grade lymphoma is rare (< 10%), and they show good prognosis and a favorable long-term survival with a complete response rate of 79% and a partial response rate of 21%. Moreover, the research of Solomonov et al. noted that primary endobronchial lymphomas show favorable response to treatment with complete remission (median follow-up period: 19-28 months) (5, 6, 21). In our series, the follow-up radiographs were available for all patients (follow-up period: 1-48 months), and none of the 5 patients who had undergone 2-chemotherapy, 2-radiotherapy, or 1-cryotherapy showed any evidence of recurrent mass or further disease progression after treatment. Furthermore, 3 of the patients demonstrated complete remission within a few months after radiotherapy or chemotherapy. Remission of both the endobronchial mass and the nodules was clearly noted on the follow-up CT scans as well as on the follow-up bronchoscopic examination in all three patients. Up to this time, these results also support the favorable outcome of primary endobronchial marginal zone B-cell lymphoma of BALT. A favorable outcome is believed to be related to the endobronchial location, which may be associated with the signs of airway obstruction typically seen, when there is a large airway-obstructing lesion, such as cough, sputum, hemoptysis, dyspnea, and wheezing (6).
According to Erbaycu et al. (19) endobronchial BALT lymphomas can affect both genders equally. There are other previous case reports which have shown female predominancy (18, 20). All patients were female in our series.
This study has a few limitations. This study was retrospective and only enrolled a small number of patients. Therefore, we could not evaluate the specific findings for differentiating between primary endobronchial marginal zone B-cell lymphoma of BALT and other endobronchial tumor. However, to the best of our knowledge, our series of 6 patients is the largest study to show the potential variety of radiologic patterns of primary endobronchial marginal zone B-cell lymphoma of BALT. We expect that a more varied radiologic manifestation may be found by examining larger groups of patients. In addition, we did not determine the exact natural course of primary endobronchial marginal zone B-cell lymphoma of BALT, because the follow-up interval was relatively short. Further studies using data with longer follow-up intervals need to be used to define accurate prognosis and determine if optical management is necessary.
In conclusion, primary endobronchial marginal zone B-cell lymphoma of BALT can manifest in three distinct patterns in the tracheobronchial tree on CT scan, but the solitary intraluminal nodule is the primary pattern. It can be considered as the differential diagnosis of endobronchial lesions in the clinical setting, if there is no widely disseminated disease or lung parenchymal involvement, homogeneous and mild FDG uptake on PET scan in the middle-aged women.
Figures and Tables
Fig. 1
Endobronchial marginal zone B-cell lymphoma of BALT in left upper lobar bronchus of 56-year-old woman who presented with 3-month history of dyspnea (patient 6 in Table 1).
A, B. Enhanced CT with transverse, coronal images showing homogeneous enhancement of endobronchial mass with distal left upper lobar atelectasis and compression of left pulmonary artery. C. 18F-FDG-PET/CT image showing hypermetabolic mass at upper left lobar bronchus (maximum SUV = 5.7). D. Bronchoscopy showing endobronchial submucosal mass at distal portion of left main bronchus with near-total obstruction of left upper lobar bronchus. E, F. Light microscopic image showing diffuse infiltration of marginal zone lymphocytes with mass formation beneath bronchial epithelium and rounded groups of small- to medium-sized lymphoid cells (Hematoxylin-Eosin stain; 40 × magnification in E; 100 × magnification in F). One month after radiotherapy, endobronchial mass disappeared (not shown).
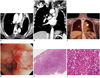
Fig. 2
Endotracheal marginal zone B-cell lymphoma of BALT in trachea of 21-year-old woman (patient 5 in Table 1).
A. Nonenhanced axial CT image obtained at level of thoracic inlet showing polypoid nodule on left side of middle trachea. B. Flexible fiberoptic bronchoscopy demonstrating nodular lesion with same smooth mucosal surface in trachea.
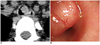
Fig. 3
Tracheobronchial marginal zone B-cell lymphoma of BALT in trachea and main stem bronchi of 42-year-old woman who presented with upper respiratory infection symptoms, including cough and sputum for 10 days (patient 2 in Table 1).
A, B. Nonenhanced transverse CT images obtained at level of aortic arch showing multiple, discontinuous, small nodular lesions along inner wall of trachea and left main bronchus. C. Flexible fiberoptic bronchoscopy demonstrating multiple, small nodular mucosal lesions with cobblestone appearance along trachea. D, E. Light microscopic image showing diffuse infiltrates of lymphoid cells within mucosa. Normal germinal center, mantle zone, and marginal zone are not shown (Hematoxylin-Eosin stain; 40 × magnification in D; 100 × magnification in E).
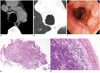
Fig. 4
Tracheobronchial marginal zone B-cell lymphoma of BALT in 54-year-old woman (patient 1 in Table 1).
A, B. Enhanced transverse CT images (5-mm-thick sections) obtained at level of carina and main bronchi showing diffuse and smooth wall thickening with homogeneous attenuation of carina and both main stem bronchi.

Table 1
Clinical Features in 7 Patients with Primary Endobronchial Marginal Zone B-Cell Lymphoma of BAT

References
1. McGuinness G, Scholes JV, Jagirdar JS, Lubat E, Leitman BS, Bhalla M, et al. Unusual lymphoproliferative disorders in nine adults with HIV or AIDS: CT and pathologic findings. Radiology. 1995. 197:59–65.
2. Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994. 84:1361–1392.
3. Kinsely BL, Mastey LA, Mergo PJ, Voytovich MC, Zander D, Almasri NM, et al. Pulmonary mucosa-associated lymphoid tissue lymphoma: CT and pathologic findings. AJR Am J Roentgenol. 1999. 172:1321–1326.
4. Li G, Hansmann ML, Zwingers T, Lennert K. Primary lymphomas of the lung: morphological, immunohistochemical and clinical features. Histopathology. 1990. 16:519–531.
5. Ahmed S, Siddiqui AK, Rai KR. Low-grade B-cell bronchial associated lymphoid tissue (BALT) lymphoma. Cancer Invest. 2002. 20:1059–1068.
6. Solomonov A, Zuckerman T, Goralnik L, Ben-Arieh Y, Rowe JM, Yigla M. Non-Hodgkin's lymphoma presenting as an endobronchial tumor: report of eight cases and literature review. Am J Hematol. 2008. 83:416–419.
7. Hoffmann M, Kletter K, Diemling M, Becherer A, Pfeffel F, Petkov V, et al. Positron emission tomography with fluorine-18-2-fluoro-2-deoxy-D-glucose (F18-FDG) does not visualize extranodal B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT)-type. Ann Oncol. 1999. 10:1185–1189.
8. Hoffmann M, Kletter K, Becherer A, Jäger U, Chott A, Raderer M. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) for staging and follow-up of marginal zone B-cell lymphoma. Oncology. 2003. 64:336–340.
9. Beal KP, Yeung HW, Yahalom J. FDG-PET scanning for detection and staging of extranodal marginal zone lymphomas of the MALT type: a report of 42 cases. Ann Oncol. 2005. 16:473–480.
10. Bae YA, Lee KS, Han J, Ko YH, Kim BT, Chung MJ, et al. Marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: imaging findings in 21 patients. Chest. 2008. 133:433–440.
11. Shin CH, Paik SH, Park JS, Kim HK, Park SI, Cha JG, et al. Primary pulmonary T-cell lymphoma: a case report. Korean J Radiol. 2010. 11:234–238.
12. Brandtzaeg P, Jahnsen FL, Farstad IN. Immune functions and immunopathology of the mucosa of the upper respiratory pathways. Acta Otolaryngol. 1996. 116:149–159.
13. Tirouvanziam R, Khazaal I, N'Sondé V, Peyrat MA, Lim A, de Bentzmann S, et al. Ex vivo development of functional human lymph node and bronchus-associated lymphoid tissue. Blood. 2002. 99:2483–2489.
14. Anaya JM, McGuff HS, Banks PM, Talal N. Clinicopathological factors relating malignant lymphoma with Sjögren's syndrome. Semin Arthritis Rheum. 1996. 25:337–346.
15. Trédaniel J, Peillon I, Fermé C, Brice P, Gisselbrecht C, Hirsch A. Endobronchial presentation of Hodgkin's disease: a report of nine cases and review of the literature. Eur Respir J. 1994. 7:1852–1855.
16. Berkman N, Lafair J, Okon E, Polliack A. Obstructive solitary bronchial non-Hodgkin's lymphoma--a rare presentation of primary extranodal disease. Leuk Lymphoma. 1992. 8:405–407.
17. McRae WM, Wong CS, Jeffery GM. Endobronchial non-Hodgkin's lymphoma. Respir Med. 1998. 92:975–977.
18. Hashemi SM, Heitbrink MA, Jiwa M, Boersma WG. A patient with endobronchial BALT lymphoma successfully treated with radiotherapy. Respir Med. 2007. 101:2227–2229.
19. Erbaycu AE, Karasu I, Ozdemirkiran FG, Yücel N, Ozsöz A, Bilgir O. Endobronchial low-grade MALT lymphoma causing unilateral hypertranslucency. Monaldi Arch Chest Dis. 2004. 61:237–240.
20. Kang JY, Park HJ, Lee KY, Lee SY, Kim SJ, Park SH, et al. Extranodal marginal zone lymphoma occurring along the trachea and central airway. Yonsei Med J. 2008. 49:860–863.
21. Nathwani BN, Anderson JR, Armitage JO, Cavalli F, Diebold J, Drachenberg MR, et al. Marginal zone B-cell lymphoma: a clinical comparison of nodal and mucosa-associated lymphoid tissue types. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1999. 17:2486–2492.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download