Abstract
Hypertrophic olivary degeneration resulting from lesions of the dento-rubro-olivary pathway, also called Guillain-Mollaret-triangle, has been described previously in a number of cases. Reports about bilateral hypertrophic olivary degeneration of the inferior olivary nuclei are very limited, and the magnetic resonance imaging findings of hypertrophic olivary degeneration in Wilson disease have not yet been described to the best of our knowledge. Herein, we present the first report of bilateral hypertrophic olivary degeneration diagnosed by magnetic resonance imaging in a patient suffering from Wilson disease.
Hypertrophic olivary degeneration (HOD) is a rare phenomenon in the dento-rubro-olivary pathway that was first described by Oppenheim in 1887 (1, 2). HOD is a unique form of transneuronal degeneration in which the inferior olivary nucleus undergoes hypertrophy followed by atrophy, as a consequence of lesions to the dento-rubro-olivary pathway; this was first defined by Guillain and Mollaret in 1931 and is thus also called Guillain-Mollaret-triangle (2, 3). Commonly, HOD occurs unilaterally and secondary to focal lesions of the Guillain-Mollaret-triangle (1, 4). We report the magnetic resonance imaging (MRI) findings in a patient suffering from Wilson disease (WD) with bilateral HOD. HOD in WD has not been described previously to the best of our knowledge.
A 23-year-old man suffering from WD was admitted to our hospital because of a deteriorating hypokinetic-rigid syndrome, increased liver enzymes, and a generalized tonic-clonic seizure that required increasing levels of chelation therapy. The patient's diagnosis of WD was made approximately 14 months prior to admission. MRI of the patient's brain at that time, revealed abnormal high signal on T2-weighted images (T2WIs) within both superior cerebellar peduncles, red nuclei, and midbrain (Fig. 1A-D). Data were acquired with a 3-T magnetic resonance scanner (Trio Tim, Siemens Healthcare, Erlangen, Germany) using a 12-channel head coil and the following sequence parameters for T2-weighted turbo spin-echo (and susceptibility weighted gradient echo) sequences: in-plane resolution Matrix = 306 × 384 (350 × 448), repetition and echo time (TR/TE): 6000/96 (28/20) ms, slice thickness = 3 (9.6 MIP) mm, field of view = 202 × 253 (178 × 228) mm2, flip angle (FA) = 120° (15°), number of excitations = 1 (1). Bilateral signal loss on both T2WI and susceptibility weighted images (SWIS) was revealed in the formatio reticularis, globus pallidus, putamen, and caudate nucleus. There was atrophy evident in the midbrain, vermis, and cerebellar hemispheres. Repeated clinical examination 6 months prior to admission had shown a cachectic patient with rigor, spastic tetraparesis accentuated in the lower extremities, severe hypomimic, and akinetic mutism without spontaneous verbal and motoric reaction. Neither palatal nor ocular myoclonus appeared at any time. Follow up MRI disclosed slight progression of the initial findings, but no signs of HOD. In the early course of hospitalization, only small improvements were achieved. Further impairment was noticed under continued chelation therapy with increasing dosage resulting from excessive mobilization of copper.
Magnetic resonance imaging after recent admission revealed progressive atrophy of the brainstem, cerebellum, cerebellar peduncles, and shrinkage of the basal ganglia. SWI showed ongoing signal loss in the putamen, head of the caudate nuclei, red nuclei, and substantia nigra on both sides. The new finding of bilaterally increased signal intensity of the inferior olivary nuclei on T2WI, accompanied by increased volumes of both nuclei, indicated a bilateral hypertrophic degeneration (Fig. 1E-H).
Hypertrophic olivary degeneration represents a unique form of transneuronal (transsynaptic) degeneration in which the inferior olivary nucleus undergoes an initial hypertrophic alteration rather than atrophy, which often occurs several years later (1, 2, 5-7). The major histologic changes of HOD are vacuolar degeneration of the enlarged neurons, hypertrophy of astrocytes, gliosis, and demyelination (2, 4, 6). On the basis of pathologic studies, macroscopic olivary enlargement has been reported as early as 3 weeks after ictus with a peak at about 8.5 months, and may resolve several years later (2, 5-7).
Birbamer et al. (8) described 3 stages of olivary degeneration in regard to the appearance on MRI: no olivary changes in the acute phase, increased signal on T2WI and proton density images corresponding to olivary hypertrophy, and persistence of high signal on T2WI with resolution of hypertrophy (3). On T2WI, a hyperintense signal of the olivary nucleus appears up to 3 weeks after disease onset, and persists for years, probably reflecting gliosis, and increased water content. On MRI, olivary enlargement is present 5 to 18 months, and may resolve approximately 3 to 4 years after the eliciting event (2, 4, 5, 8). Dinçer et al. (9) described a decrease in radial diffusivity compatible with demyelination and an increase in axial diffusivity representing neuronal hypertrophy in all components of the Guillain-Mollaret-triangle in patients with HOD.
Palatal and ocular myoclonus, dentatorubral (or Holmes') tremor, cyclic jerk of the soft palate, and other involuntary movements are the hallmark symptoms and have been described as early as 1886 (2, 7, 10).
The corners of the Guillain-Mollaret-triangle consist of the olivary nucleus, ipsilateral red nucleus, and contralateral dentate nucleus. The edges are formed by projections of the central tegmental tract between olivary nucleus and red nucleus, the superior cerebellar peduncle between red nucleus and dentate nucleus, and the inferior cerebellar peduncle connecting the dentate nucleus with the olivary nucleus (1, 4). Lesions within the neuronal network of the Guillain-Mollaret-triangle causing HOD are commonly unilateral and may result from hemorrhage due to vascular malformation, trauma, surgical intervention or hypertension, tumor, ischaemia, or inflammation (1, 3-5, 11). Unilateral HOD is observed if the lesion affects the ispilateral central tegmental tract, the contralateral dentate nucleus, or superior cerebellar peduncle, respectively (1, 4).
Reports of bilateral HOD are extremely rare and limit our knowledge exclusively to focal lesions (3, 7, 10, 11). Gerace et al. (10) showed a bilateral HOD from a solitary ischaemic lesion located in the midbrain and corresponding to the decussed region of the superior cerebellar peduncle. Auffray-Calvier et al. (11) described 3 cases of bilateral HOD resulting from hemorrhage and tumor surgery. Causative lesions were identified in both dentate nuclei, in both central tegmental tracts, and in the ipsilateral dentate nucleus and central tegmental tract, respectively. In addition, bilateral olivary degeneration may occur if both the superior cerebellar peduncle and the central tegmental tract are involved (4, 12).
Asal et al. (3) outlined that lesions such as ischemia, tumors, demyelinating and inflammatory diseases could cause hyperintensity of the olivary nucleus on T2WI and have to be considered in the differential diagnosis. An involvement of the contralateral cerebellum or ipsilateral brainstem is a significant diagnostic criterion for olivary hypertrophy (3). Wallerian degeneration, amyotrophic lateral sclerosis, and adrenoleukodystrophy demonstrate prior hyperintensity on T2WI of the corticospinal tract and the rostral segment of the medulla rather than on the olivary nucleus (7). The absence of contrast enhancement helps to differentiate between a tumoral lesion or an infection (3).
To the best of our knowledge, there has been no previous report of bilateral HOD resulting from a genetic disorder. WD is considered to be an extrapyramidal disorder with abnormalities of the grey matter nuclei and atrophy (13). Beside the latter-mentioned, its neuroimaging spectrum involves the extrapyramidal white matter, dentatorubrothalamic, and pontocerebellar tract, which form the cortico-ponto-cerebello-dentato-rubro-thalamo-cortico circuit. The dentatorubrothalamic tract originates in the dentate nucleus and decussates as superior cerebellar peduncle to the red nucleus before terminating in the ventrolateral nucleus of the thalamus. This extrapyramidal tract can secondary be damaged next to the more severely affected extrapyramidal grey nuclei (13). We found bilateral HOD resulting from widespread, non-focal neuronal damage involving all constituents of the Guillain-Mollaret-triangle beside the abnormal supra-and infra-tentorial MRI findings within the scope of the underlying disease. Neuroimaging findings in WD represent the neuropathologic spectrum of spongy to cystic degeneration of grey matter nuclei, capillary endothelial swelling, pontine myelinolysis, gliosis, and demyelination, as well as accumulation of iron and copper (13-15).
The most frequent affected sites on MRI in WD are the basal ganglia with an abnormal low signal intensity on SWI, but increased signal intensity on T2WI, which reflects the formerly mentioned neuropathologic findings. In particular, the putamina show an increased signal intensity in the outer rim on T2WI. Second, the most involved are pons, midbrain, cortical white matter, and cerebellum with hyperintense signal changes on T2WI. Furthermore supra- and infra-tentorial atrophy can be present (16).
The severe course and progression of the disease in the presented case may have contributed to the propagation of structural tissue damage even within infra-tentorial compartments, including the constituents of the Guillain-Mollaret-triangle, beside the extrapyramidal grey nuclei and (extra-)pyramidal white matter tracts (13), and underline the potentially non-selective affection of the central nervous system by WD.
Figures and Tables
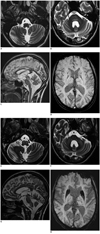
Fig. 1
Bilateral hypertrophic olivary degeneration in Wilson disease (WD).
23-year-old man suffering from WD with initial MRI findings. A. Transverse T2WI (TR/TE: 6000/96 ms, FA = 120°) at level of medulla oblongata without signs of hypertrophy or increased signal intensity of inferior olivary nuclei on both sides. B. Transverse T2WI at level of pons: symmetrical hyperintensity of pontine tegmentum. C. Midsagittal T2WI: hyperintense midbrain with atrophy of cerebellum. D. Transverse SWI (TR/TE: 28/20 ms, FA = 15°) at level of basal ganglia shows low signal intensity of heads of caudate nuclei and putamina. SWI = susceptibility weighted image, T2WI = T2-weighted image
MRI findings of same patient one year later. E. Transverse T2WI (TR/TE: 6000/96 ms, FA = 120°) at level of medulla oblongata: bilateral hyperintense and hypertrophic inferior olivary nuclei (arrows). F. Transverse T2WI at level of pons: symmetrical hyperintensity and atrophy of medial cerebellar peduncles and pons. G. Parasagittal T2WI: hyperintense midbrain, pons, medulla; atrophy of superior cerebellar peduncle (arrow) and cerebellum. H. Transverse SWI (TR/TE: 28/20 ms, FA = 15°) at level of basal ganglia: considerable volume and ongoing signal loss in heads of caudate nuclei and putamina. SWI = susceptibility weighted image, T2WI = T2-weighted image
References
1. Hornyak M, Osborn AG, Couldwell WT. Hypertrophic olivary degeneration after surgical removal of cavernous malformations of the brain stem: report of four cases and review of the literature. Acta Neurochir (Wien). 2008. 150:149–156. discussion 156.
2. Kitajima M, Korogi Y, Shimomura O, Sakamoto Y, Hirai T, Miyayama H, et al. Hypertrophic olivary degeneration: MR imaging and pathologic findings. Radiology. 1994. 192:539–543.
3. Asal N, Yılmaz O, Turan A, Yiğit H, Duymuş M, Tekin E. Hypertrophic olivary degeneration after pontine hemorrhage. Neuroradiology. 2012. 54:413–415.
4. Goyal M, Versnick E, Tuite P, Cyr JS, Kucharczyk W, Montanera W, et al. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol. 2000. 21:1073–1077.
5. Phatouros CC, McConachie NS. Hypertrophic olivary degeneration: case report in a child. Pediatr Radiol. 1998. 28:830–831.
6. Goto N, Kaneko M. Olivary enlargement: chronological and morphometric analyses. Acta Neuropathol. 1981. 54:275–282.
7. Salamon-Murayama N, Russell EJ, Rabin BM. Diagnosis please. Case 17: hypertrophic olivary degeneration secondary to pontine hemorrhage. Radiology. 1999. 213:814–817.
8. Birbamer G, Buchberger W, Felber S, Aichner F. MR appearance of hypertrophic olivary degeneration: temporal relationships. AJNR Am J Neuroradiol. 1992. 13:1501–1503.
9. Dinçer A, Özyurt O, Kaya D, Koşak E, Öztürk C, Erzen C, et al. Diffusion tensor imaging of Guillain-Mollaret triangle in patients with hypertrophic olivary degeneration. J Neuroimaging. 2011. 21:145–151.
10. Gerace C, Fele MR, Luna R, Piazza G. Neurological picture. Bilateral hypertrophic olivary degeneration. J Neurol Neurosurg Psychiatry. 2006. 77:73.
11. Auffray-Calvier E, Desal HA, Naudou-Giron E, Severin-Fontana S, Cavenaile-Dolez H, Stefan A, et al. [Hypertrophic olivary degeneration. MR imaging findings and temporal evolution]. J Neuroradiol. 2005. 32:67–72.
12. Tsui EY, Cheung YK, Mok CK, Yuen MK, Chan JH. Hypertrophic olivary degeneration following surgical excision of brainstem cavernous hemangioma: a case report. Clin Imaging. 1999. 23:215–217.
13. van Wassenaer-van Hall HN, van den Heuvel AG, Jansen GH, Hoogenraad TU, Mali WP. Cranial MR in Wilson disease: abnormal white matter in extrapyramidal and pyramidal tracts. AJNR Am J Neuroradiol. 1995. 16:2021–2027.
14. Magalhaes AC, Caramelli P, Menezes JR, Lo LS, Bacheschi LA, Barbosa ER, et al. Wilson's disease: MRI with clinical correlation. Neuroradiology. 1994. 36:97–100.
15. Sinha S, Taly AB, Ravishankar S, Prashanth LK, Vasudev MK. Central pontine signal changes in Wilson's disease: distinct MRI morphology and sequential changes with de-coppering therapy. J Neuroimaging. 2007. 17:286–291.
16. King AD, Walshe JM, Kendall BE, Chinn RJ, Paley MN, Wilkinson ID, et al. Cranial MR imaging in Wilson's disease. AJR Am J Roentgenol. 1996. 167:1579–1584.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download