Abstract
Objective
To evaluate the temporal evolution and diagnostic values of the diffusion tensor imaging (DTI) and the high b value diffusion weighted imaging (DWI) in the early permanent and transient cerebral ischemia.
Materials and Methods
For permanent or 30-minute transient-ischemia induced 30 rats, DTI and DWIs at both high b (b = 3000 s/mm2) and standard b value (b = 1000 s/mm2) were obtained at the following conditions: at 15, 30, 45, 60 minutes after the occlusion of what for hyperacute permanent ischemia; at 1, 3, 5, 7, 9 hours after the occlusion for acute permanent ischemia; and at 15 minutes before reperfusion, 0.5, 2.5, and 24 hours after reperfusion for transient ischemia. The diffusion parameters and their ratios were obtained and compared between different b values, and among different time points and groups, respectively.
Results
For both b values, the apparent diffusion coefficient (ADC) ratio decreased for first three hours, and then slightly increased until 9 hours after the occlusion during a gradual continuous increase of DWI signal intensity (SI) ratio, with excellent correlation between ADC ratios and DWI SI ratios. The DWI showed a higher contrast ratio, but the ADC map showed a lower contrast ratio for permanent ischemia at high b value than at standard b value. Fractional anisotropy (FA) increased for 1 hour, then gradually decreased until 9 hours after the occlusion in permanent ischemia and showed transient normalization and secondary decay along with change in ADC in transient ischemia.
With the advent of increasingly higher field strength along with recent progress in the MR gradient technology, increased diffusion sensitivity has now become possible without significant loss of signal to noise ratio (SNR) (1). For applications to the brain, as the diffusion of brain tissue has been known to show biexponential decay with increasing b-value, high b value diffusion weighted imaging (DWI) has been applied to a few diseases (2-4). However, its clinical benefit in the evaluation of cerebral infarct has often been debated (3, 5-7). In addition, it is still unknown how differently high b value DWI evolutes with time, which may give basic information about the time window for the thrombolytic treatment, and how beneficial it is in the evaluation of early permanent and transient ischemia as compared with standard b value DWI.
Diffusion tensor imaging (DTI), which captures anisotropic diffusion and provides structural information, has also been used to evaluate many diseases including cerebral infarct (8-10). However, as of yet, we do not know exactly how fractional anisotropy (FA) changes according to time, in relation to the apparent diffusion coefficient (ADC) in early permanent and transient ischemias (11-13). Hence, reevaluation of the diffusion parameters of early permanent and transient ischemia using these new modalities is warranted.
In this study, we present the temporal changes of DWI signal intensity (SI) and ADC at high b value and FA, and evaluate their diagnostic values in comparison with standard b value DWI, in rat models with early permanent and transient cerebral ischemia.
The Animal Research Committee of Seoul National University Hospital approved this study. Forty five male Sprague-Dawley rats (Daehan Biolink Co., Eumseong, Korea) were initially allocated to the hyperacute permanent ischemia group (n = 15), the acute permanent ischemia group (n = 15), and the 30 minute transient ischemia group (n = 15). Because of the technical limitations including death during ischemia (n = 9) and failure of inducing ischemia (n = 6), the study enrolled 30 male Sprague-Dawley rats (636 ± 140 g) comprised of 22 rats of the permanent ischemia group (9 hyperacute and 13 acute) and 8 rats of the 30 minute transient ischemia group.
Arbitrarily, we decided the time point between the hyperacute group and the acute group as 1 hour after occlusion of middle cerebral artery (MCA), referring to Neumann-Haefelin et al. (14) results that the occlusion of MCA until 1 hour could show reversibility in a rat model although the reversibility was transient.
In all rats, MCA occlusion was induced by advancing a silicone coated monofilament nylon suture (4-0 Ethilon, Ethicon Inc., Someville, NJ, USA) tip rounded by flame heating to the anterior cerebral artery through the carotid bifurcation, referring to the previously described intraluminal suture occlusion models in rats (15, 16). For the transient ischemia group, the rats were reperfused by withdrawing the suture after 30 minute occlusion. Anesthesia was induced with a 1 : 1 mixture of Ketamine hydrochloride (5 mg/100 g) and xylazine hydrochloride (1 mg/100 g) during surgery and imaging scanning. Body temperature was monitored with a rectal probe and maintained at 37.5 ± 0.5℃ with a warm air circulation system. Pulse oximetry was used to monitor oxygen saturation and heart rate. Respiration and heart rate were also monitored, as all animals were allowed to breathe spontaneously throughout the experiment. All of the physiological parameters of both groups remained within the normal range.
Immediately after occlusion, the rats were transferred to a 3.0 T MRI scanner (Signa; GE Medical Systems, Milwaukee, WI, USA). DTI and high and standard b value DWIs were performed at 15, 30, 45, 60 minutes for the hyperacute permanent ischemia group, at 1, 3, 5, 7, and 9 hours for the acute permanent ischemia group, and at 15 minute before reperfusion, 0.5, 2.5, and 24 hours after reperfusion for the 30 minute transient ischemia group. All MR images were obtained using 8 channel head coil with the following parameters: FOV = 8 × 8 cm, matrix = 64 × 128, and section thickness = 3 mm. With TR/TE = 1000/60 ms and 1 NEX, the perfusion-weighted imaging (PWI) was performed using spin-echo echo planar imaging to follow the passage of a bolus injection of 0.3 mmol/kg gadolinium dimeglumine (Magnevist, Schering AG, Berlin, Germany) through the tail vein.
Imaging parameters for DTI were as follows: b = 0 and 700 s/mm2, 6 directions, TR/TE = 6000/78.9 ms, and NEX = 32. For high b value: b = 0 and 3000 s/mm2, TR/TE = 6000/99.6 ms, and NEX = 15. For standard b value: b = 0 and 1000 s/mm2, TR/TE = 6000/79 ms, and NEX = 8. ADC maps were generated by a two-point linear fit approach on a pixel basis. As the increase of b value results in the loss of SNR at DWI, we increased NEX to be greater at the high b value than at the standard b value (6).
Successful occlusion was assessed by the region of interest (ROI) analysis of SI on PWI 10 minutes after occlusion, and successful reperfusion was assessed on PWI 10 minutes after reperfusion. Following the last MRI protocol, the rats were deeply anaesthetized and euthanized by decapitation. The brains were then removed from the skull and incubated for 30 minutes in a 2% solution of triphenyltetrazolium chloride at 37℃, as well as fixation by immersion in a 10% buffered formalin solution. The brains were then inspected for evidence of hemorrhage and evaluated for adequate infarct.
Because hemorrhage was found in 2 rats and adequate infarction was not found in 4 rats, 6 rats were regarded as failures of inducing ischemia and were excluded in the analysis.
To measure FA, ADC, and DWI SI, two circular ROIs (0.87 ± 0.16 cm2) of the same size were chosen at the ADC map and copied to the corresponding maps, one in the center of the ischemic lesion (caudoputamen) and the other in the contralateral normal tissue at the level of anterior commissure. The SNR of DWI was obtained by dividing DWI SI by the standard deviation of background SI as measured on the ROI (2.25 ± 0.32 cm2). Values at the ipsilateral ischemia were normalized by contralateral normal values to produce FA, ADC, and DWI SI ratios.
Contrast ratios and degrees of decrease were calculated as follows:
Contrast ratios at b = 1000 or 3000 s/mm2: |SIischemia - SInormal| / SInormal [1]
Degrees of decrease in ischemia or normal tissue when b values were increased from:
1000 s/mm2 to 3000 s/mm2: |SIb1000 - SIb3000| / SIb1000 × 100 (%) [2]
where SI is ADC or SNR of DWI.
Continuous variables were presented as means ± standard deviations. Differences of all parameters and their ratios between different b values, different time points, and different groups were calculated using the paired t test or student t test as appropriate. Pearson correlation coefficient analysis was performed between the different b value DWIs and ADCs. A p < 0.05 was considered to indicate statistical significance. All analyses were performed using SPSS version 13 software for Windows (SPSS Inc, Chicago, IL, USA).
There were no statistical differences in the values all of 1 hour parameters (FA, SNR of DWI and ADC at both b values) and their ratios between the hyperacute permanent ischemia group and the acute permanent ischemia group.
Table 1 presents the serial ADC changes at b = 1000 and b = 3000 s/mm2 during the early permanent ischemia. At all time points, both ADCs and SNRs of DWI SI decreased when b-values increased from 1000 s/mm2 to 3000 s/mm2 in both ischemia and normal tissue (p < 0.0001).
Figures 1 and 2 illustrate the characteristic MRI patterns and the average changes in SI ratios on ADC maps, DWI, and FA maps in the permanent ischemia.
At both b values, ADC ratios decreased for 3 hours and then rebounded until 9 hours after occlusion. ADC ratios were higher at b = 3000 s/mm2 than at b = 1000 s/mm2 at all time points (p < 0.0001). Conversely, contrast ratios of ADC maps at b = 3000 s/mm2 were lower than those at b = 1000 s/mm2 at all time points (0.25 ± 0.10 at b = 3000 s/mm2 vs. 0.38 ± 0.09 at b = 1000 s/mm2, p < 0.0001). The degrees of ADC decrease in ischemia were lower than those in normal tissue at all time points (25.9 ± 4.3% in ischemia vs. 38.63 ± 3.27% in normal tissue, p < 0.0001).
At both b values, DWI SI ratios increased continuously from 15 minutes until 9 hours after occlusion. DWI SI ratios were higher at b = 3000 s/mm2 than at b = 1000 s/mm2 at all time points, with statistical significances from 1 to 9 hours (p = 0.0048 at 1 hour, < 0.0001 at 3 to 9 hours). Contrast ratios of DWI were higher at b = 3000 s/mm2 than b = 1000 s/mm2 at all time points (0.67 ± 0.34 at b = 3000 s/mm2 vs. 0.54 ± 0.24 at b = 1000 s/mm2), with statistical significances from 30 minutes to 9 hours (p < 0.0001). The degree of DWI SI decrease in ischemia was lower than that of normal tissue at all time points (53.0 ± 2.46% in ischemia vs. 56.47 ± 2.88% in normal tissue), with statistical significances from 30 minutes to 9 hours (p < 0.0001).
The Pearson correlation study showed excellent correlation in ADC ratios and DWI SI ratios between both b values (Fig. 3).
Figures 4 and 5 illustrate the characteristic MRI patterns and the average changes in SI ratios on ADC maps, DWI, and FA maps in the 30 minute transient ischemia.
After 30 minute transient ischemia, DWI SI ratios and ADC ratios at both b values were transiently normalized to the contralateral normal tissue level at 30 minutes and 2.5 hours after reperfusion. At 24 hours after reperfusion, the DWI SI ratios at both b values reincreased and the ADC ratios at both b values redecreased.
The FA increased for 1 hour, then gradually decreased until 9 hours after occlusion in the permanent ischemia.
As for transient ischemia, FA ratios showed initial elevation, then transiently normalized to the contralateral normal tissue level at 30 minutes after reperfusion and maintained this level until 2.5 hours after reperfusion. There was a significant decrease in FA ratio at 30 minutes after reperfusion in the 30 minute transient ischemia group compared to that at 1 hour in the permanent ischemia group, which were at the same time points, 1 hour after initial occlusion (1.01 ± 0,08 vs. 1.23 ± 0.22; p = 0.0128). At 24 hours after reperfusion, the FA ratio decreased as the ADC ratio decreased, suggesting secondary decay.
In the present study, the 3 hour mark was the time point with the lowest ADC value after occlusion in the early permanent rat ischemia, and is concordant with the results of several previous studies (17, 18).
We also observed that when the b-values increased, there were decreases in the ADC and SNR of DWI in both the ischemia and normal tissues, which was also reported in a study by Meyer et al. (5). This can be explained by the suggestion that ADC decreases biexponentially rather than monoexponentially along with the b value increases (1, 2).
The ADC ratio and DWI SI ratio at b = 3000 s/mm2 were higher than those at b = 1000 s/mm2. And while the contrast ratio of ADC maps at b = 3000 s/mm2 was lower than that at b = 1000 s/mm2, the contrast ratio of DWI at b = 3000 s/mm2 was higher than that at b = 1000 s/mm2.
These observations can be postulated as follows:
As the degree of ADC decrease in ischemia was smaller than that in normal tissue from the results section,
(ADCischemia-b1000 - ADCischemia-b3000) / ADCischemia-b1000 < (ADCnormal-b1000 - ADCnormal-b3000) / ADCnormal-b1000,
It can be shown as the following inequality:
ADCischemia-b1000 / ADCnormal-b1000 < ADCischemia-b3000 / ADCnormal-b3000
Such means that ADC ratio at b = 3000 s/mm2 is larger than that at b = 1000 s/mm2. This was also shown in Figure 2.
From here, we can deduce the following:
1 - ADCischemia-b1000 / ADCnormal-b1000 > 1 - ADCischemia-b3000 / ADCnormal-b3000
Because ADC in normal tissue is larger than that in ischemia at both b values, the following formula can be stated:
|ADCnormal-b1000 - ADCischemia-b1000| / ADCnormal-b1000 > |ADCnormal-b3000 - ADCischemia-b3000| / ADCnormal-b3000
In other words, the contrast ratio of ADC map at b = 1000 s/mm2 is larger than that at b = 3000 s/mm2.
As with the previous mathematical deduction, as the degree of DWI SI decrease in ischemia was smaller than that in normal tissue from the results section,
(DWIischemia-b1000 - DWIischemia-b3000) / DWIischemia-b1000 < (DWInormal-b1000 - DWInormal-b3000) / DWInormal-b1000
It can be shown as the following inequality:
DWIischemia-b1000 / DWInormal-b1000 < DWIischemia-b3000 / DWInormal-b3000
Such means that DWI ratio at b = 3000 s/mm2 is larger than that at b = 1000 s/mm2. This was also shown in Figure 2.
From here, we can deduce the following:
1 - DWIischemia-b1000 / DWInormal-b1000 > 1 - DWIischemia-b3000 / DWInormal-b3000
As DWI SI in normal tissue is smaller than that in ischemia at both b values, the following formula can be stated:
|DWInormal-b1000 - DWIischemia-b1000| / DWInormal-b1000 < |DWInormal-b3000 - DWIischemia-b3000| / DWInormal-b3000
In other words, the contrast ratio of DWI at b = 1000 s/mm2 is lower than that at b = 3000 s/mm2.
From the above inequalities, we can reason that the higher contrast ratio of DWI and lower contrast ratio of ADC map at higher b value are due to the smaller degrees of ADC and DWI decrease in ischemia than those in the normal tissue.
Although the reason for this difference between the normal tissue and ischemia may not be easily explained, it may be due to the slow diffusing component fraction, which may be reflected more on the higher b value imaging, being smaller in the normal tissue than in ischemia, as Brugières et al. (19) presented.
However, the ADCs at both the high and standard b values showed a similar pattern of chronological change except for some differences in the ADC value. This may be because the high b value ADC calculated on two-point linear fit approach failed to reflect the selectively slow diffusing component fraction.
Burdette et al. (20) suggested that a higher diffusion gradient increased the relative contribution of ADC to the overall MR SI, which also meant a decreased T2 shine-through phenomenon.
The reason for the contrast ratios on DWI at the high b value being higher than that at the standard b value, although the contrast ratio on ADC maps at the high b value are lower, can be postulated based on the following formula:
DWI SI = S0 × e-bxADC [3]
where S0 is SI in b = 0 s/mm2, and b is the diffusion-weighting factor.
As DWI SI is influenced by both the diffusion gradient and ADC, the higher contrast ratio on DWI can be due to the effects of the increased diffusion gradient surpassing the decreased ADC difference, when b-values were increased. Although high b value imaging have the clinical benefits in the detection and evaluation of the extent of hyperacute and acute ischemia in addition to the higher contrast ratio on DWI, it has tradeoffs such as inferior SNR, change of gray to white matter SI ratio, and a low contrast ratio on ADC maps, as shown in the present study (3, 5-7). Therefore, the b value in the high b value imaging should be optimized for the specific purpose of each evaluation.
The initial transient elevation of FA ratio seen in the hyperacute ischemia in the present study is in agreement with the results of the previous studies (11, 21). It has been explained that the cellular expansion and tortuosity of the extracellular space cause a limitation of the extracellular water motion in the initial early ischemia. It is followed by the loss of cellular membranous integrity and the expansion of extracellular space with the over-accumulation of water, ultimately resulting in the subsequent decrease of FA (11-13).
Considering the results of Bae et al. (22) study which presented the reduction of FA due to the injection of contrast material in the brain tumor, the possibility of the contrast material's influence on the initial FA in the present study may not be excluded but validated with the future study in the ischemia. Transient reversibility and secondary ADC decay of cerebral ischemia has been explained by many causes, such as neuronal cell death caused by delayed depletion of energy, programmed cell death, and reperfusion injury (23). This study showed that FA can be transiently normalized from the initially elevated value, although it is decayed on the 1 day follow-up like the secondary decay of ADC.
The traditional DWI-PWI mismatch has been reported to have some limitations in the evaluation of ischemia (24). The observations that FA increases in the hyperacute ischemia, with a continuous decrease of ADC and integrative interpretation of reversible ADC and FA, may provide a new breakthrough for the evaluation of ischemic penumbra in the early ischemia. Further studies are needed on this aspect.
The present study has the following limitations. First, we divided the permanent group of the study into two separate groups, as the frequent repeated imaging acquisition while keeping the rats alive was impossible at the given facilities. Thus by separating the two permanent groups, we were able to obtain hyperacute information with intervals of 15 minutes and acute information with total observation time of up to 9 hour. Second, the use of a clinical MR machine may be a limitation. However, the ischemic areas on the clinical MR were well depicted as compared with the postmortem evaluation. Chen et al. (25) have also suggested that the performance of certain stroke-related research in rats was feasible with the clinical MR imagers. Third, different TEs at b = 1000 s/mm2 and b = 3000 s/mm2 may have been another limitation. However, the TEs used in the study were minimum TEs and were optimized for the 2 b-values. According to some reports, the ADC of brain water was found to be approximately independent of TE (1, 2).
In summary, this study presents the time evolutions of DWI SI and ADC ratios at high b value and FA ratio in early permanent and transient ischemia. Although high b value DWI may reflect biexponential decay, it has shown similar chronological patterns of DWI and ADC map on two-point linear fit approach in the early cerebral ischemia as the standard b value DWI. At high b value, DWI showed higher contrast ratio, but ADC map showed lower contrast ratio than the standard b value in the early permanent ischemia. In addition to the initial elevation in early ischemia, FA shows transient reversibility similar to ADC, the clinical significance of which needs to be verified.
Figures and Tables
Fig. 1
Serial MRI patterns of hyperacute (A) and acute (B) ischemia on DWIs and ADC maps, at b = 1000 s/mm2 and b = 3000 s/mm2; and FA map in two representative rats, with rat brain (C) stained with triphenyltetrazolium chloride 9 hours after occlusion.
DWI hyperintensity and ADC hypointensity at both b values are seen in left cerebral hemisphere from 15 minutes to 9 hours. Compared with lesion contrast at b = 1000 s/mm2, that at b = 3000 s/mm2 is higher on DWI but lower on ADC map. FA in left cerebral hemisphere is higher than normal tissue for 1 hour. It decreases to value lower than that of normal tissue at 9 hours. Rat brain stained with triphenyltetrazolium chloride shows pale brain area, suggesting adequate infarct of middle cerebral artery. ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, FA = fractional anisotropy
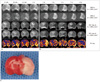
Fig. 2
Time curves of ratios of DWI signal intensity, ADC, and FA in hyperacute and acute permanent ischemia groups.
Values of 1 hour parameters are mean of both hyperacute and acute permanent ischemia groups (arrows). Values at ipsilateral ischemia were normalized by contralateral normal values to produce ratios of all parameters. Error bars reflect standard error of mean. ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, FA = fractional anisotropy, SI = signal intensity

Fig. 3
Scatterplots of correlation in ADC ratios and DWI SI ratios between b = 3000 s/mm2 and b = 1000 s/mm2.
Strong correlation in ADC ratios (A) and DWI SI ratios (B) between both b values is seen. ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, SI = signal intensity

Fig. 4
Serial MRI patterns of transient ischemia on DWI and ADC maps at b = 1000 s/mm2 and b = 3000 s/mm2, and FA map.
After 30 minute transient ischemia, normalizations of DWI hyperintensity and ADC hypointensity at both b values, as well as normalization of FA hyperintensity at left caudoputamen area are seen during first 2.5 hours, with the exception of some peripheral portions. ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, FA = fractional anisotropy
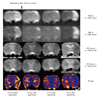
Fig. 5
Time curves in transient ischemia group and Comparison of ADC and FA in permanent and transient groups.
(A) Time curves of DWI signal intensity, ADC, and FA ratios in transient ischemia group. After 30 minute transient cerebral ischemia, initial high DWI SI and FA ratios reach plateau of near 1 during first 2.5 hours after reperfusion, followed by reincrease of DWI SI ratio and decrease of FA ratio at 24 hours. Initial low ADC ratio reaches plateau of near 1 during first 2.5 hours after reperfusion, followed by redecrease of ADC ratio at 24 hours. (B) Comparison of ADC and FA in permanent and transient groups. At 30 minutes after 30 minute occlusion in transient ischemia group, which is comparable to 1 hour after occlusion in permanent ischemia group, FA decreased and ADC increased significantly, resulting in normalized values. ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, FA = fractional anisotropy, SI = signal intensity

References
1. DeLano MC, Cooper TG, Siebert JE, Potchen MJ, Kuppusamy K. High-b-value diffusion-weighted MR imaging of adult brain: image contrast and apparent diffusion coefficient map features. AJNR Am J Neuroradiol. 2000. 21:1830–1836.
2. Niendorf T, Dijkhuizen RM, Norris DG, van Lookeren Campagne M, Nicolay K. Biexponential diffusion attenuation in various states of brain tissue: implications for diffusion-weighted imaging. Magn Reson Med. 1996. 36:847–857.
3. Toyoda K, Kitai S, Ida M, Suga S, Aoyagi Y, Fukuda K. Usefulness of high-b-value diffusion-weighted imaging in acute cerebral infarction. Eur Radiol. 2007. 17:1212–1220.
4. Seo HS, Chang KH, Na DG, Kwon BJ, Lee DH. High b-value diffusion (b = 3000 s/mm2) MR imaging in cerebral gliomas at 3T: visual and quantitative comparisons with b = 1000 s/mm2. AJNR Am J Neuroradiol. 2008. 29:458–463.
5. Meyer JR, Gutierrez A, Mock B, Hebron D, Prager JM, Gorey MT, et al. High-b-value diffusion-weighted MR imaging of suspected brain infarction. AJNR Am J Neuroradiol. 2000. 21:1821–1829.
6. Burdette JH, Elster AD. Diffusion-weighted imaging of cerebral infarctions: are higher B values better? J Comput Assist Tomogr. 2002. 26:622–627.
7. Kim HJ, Choi CG, Lee DH, Lee JH, Kim SJ, Suh DC. High-b-value diffusion-weighted MR imaging of hyperacute ischemic stroke at 1.5T. AJNR Am J Neuroradiol. 2005. 26:208–215.
8. Melhem ER, Mori S, Mukundan G, Kraut MA, Pomper MG, van Zijl PC. Diffusion tensor MR imaging of the brain and white matter tractography. AJR Am J Roentgenol. 2002. 178:3–16.
9. Ahn S, Lee SK. Diffusion tensor imaging: exploring the motor networks and clinical applications. Korean J Radiol. 2011. 12:651–661.
10. Tong T, Zhenwei Y, Xiaoyuan F. Transient ischemic attack and stroke can be differentiated by analyzing the diffusion tensor imaging. Korean J Radiol. 2011. 12:280–288.
11. Yang Q, Tress BM, Barber PA, Desmond PM, Darby DG, Gerraty RP, et al. Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke. 1999. 30:2382–2390.
12. Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, et al. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999. 212:785–792.
13. Zelaya F, Flood N, Chalk JB, Wang D, Doddrell DM, Strugnell W, et al. An evaluation of the time dependence of the anisotropy of the water diffusion tensor in acute human ischemia. Magn Reson Imaging. 1999. 17:331–348.
14. Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000. 31:1965–1972. discussion 1972-1973.
15. Li F, Han S, Tatlisumak T, Carano RA, Irie K, Sotak CH, et al. A new method to improve in-bore middle cerebral artery occlusion in rats: demonstration with diffusion- and perfusion-weighted imaging. Stroke. 1998. 29:1715–1719. discussion 1719-1720.
16. Gerriets T, Li F, Silva MD, Meng X, Brevard M, Sotak CH, et al. The macrosphere model: evaluation of a new stroke model for permanent middle cerebral artery occlusion in rats. J Neurosci Methods. 2003. 122:201–211.
17. Bardutzky J, Shen Q, Henninger N, Bouley J, Duong TQ, Fisher M. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke. 2005. 36:2000–2005.
18. Dijkhuizen RM, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Regional assessment of tissue oxygenation and the temporal evolution of hemodynamic parameters and water diffusion during acute focal ischemia in rat brain. Brain Res. 1997. 750:161–170.
19. Brugières P, Thomas P, Maraval A, Hosseini H, Combes C, Chafiq A, et al. Water diffusion compartmentation at high b values in ischemic human brain. AJNR Am J Neuroradiol. 2004. 25:692–698.
20. Burdette JH, Elster AD, Ricci PE. Acute cerebral infarction: quantification of spin-density and T2 shine-through phenomena on diffusion-weighted MR images. Radiology. 1999. 212:333–339.
21. Liu Y, D'Arceuil HE, Westmoreland S, He J, Duggan M, Gonzalez RG, et al. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke. 2007. 38:138–145.
22. Bae MS, Jahng GH, Ryu CW, Kim EJ, Choi WS, Yang DM. Effect of intravenous gadolinium-DTPA on diffusion tensor MR imaging for the evaluation of brain tumors. Neuroradiology. 2009. 51:793–802.
23. Kidwell CS, Saver JL, Starkman S, Duckwiler G, Jahan R, Vespa P, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002. 52:698–703.
24. Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003. 34:2729–2735.
25. Chen F, Suzuki Y, Nagai N, Peeters R, Coenegrachts K, Coudyzer W, et al. Visualization of stroke with clinical MR imagers in rats: a feasibility study. Radiology. 2004. 233:905–911.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download