Abstract
Objective
To evaluate the efficacy of computer tomography (CT)-guided core needle biopsy (CNB) in the diagnosis of deep suprahyoid lesions in patients with treated head and neck cancers.
Materials and Methods
Between December, 2003 and May, 2011, 28 CT-guided CNBs were performed in 28 patients with deep suprahyoid head and neck lesions. All patients had undergone treatment for head and neck cancers. Subzygomatic, paramaxillary, and retromandibular approaches were used. The surgical results, response to treatment, and clinical follow-up were used as the diagnostic reference standards.
Results
All biopsies yielded adequate specimens for definitive histological diagnoses. A specimen from a right parapharyngeal lesion showed atypia, which was deemed a false negative diagnosis. Diagnostic accuracy was 27/28 (96.4%). Two minor complications were encountered: a local hematoma and transient facial palsy. Between the 18 or 20 gauge biopsy needles, there was no statistical difference in the diagnostic results.
Conclusion
CT-guided core needle biopsy, with infrequent and minor complications, is an accurate and efficient method for the histological diagnosis of deep suprahyoid lesions in post-treated head and neck cancer patients. This procedure can preclude an unnecessary surgical intervention, especially in patients with head and neck cancers.
Deep lesions in the head and neck area are often clinically inaccessible, thus, obtaining a tissue specimen can be a challenging matter (1). Surgical open biopsy is commonly used to achieve the diagnosis, but it is invasive and has notable risks. Ultrasound (US) guidance has been generally advocated for the biopsy of most head and neck lesions, especially for the superficial areas (2). However, this technique has limited utility for lesions in the deep suprahyoid region because the underlying intervening osseous and air-containing structures may contribute to a limited ultrasound window. CT is the imaging modality of choice for biopsies of deep-seated head and neck lesions (3). The reported diagnostic accuracy of CT-guided fine needle aspiration (FNA) is 86-88% (4, 5). However, the diagnostic efficacy of FNA depends significantly on the cytology expertise, and can be biased by the specimen quantity and quality (6). Alternatively, core needle biopsy (CNB) has been considered efficient and safe for tissue diagnosis of head and neck lesions. It can provide quality specimens for histopathologic and immunochemical studies (2, 7, 8). However, CT-guided CNB can be technically challenging for deep head and neck areas due to major vessels and nerves that often obscure the path of the needle (3). Further, it can be more difficult for patients that underwent treatment for head and neck cancers since the prior surgery or radiotherapy often distorts the original anatomic structure. Only few studies have reported the results of CT-guided CNB in the diagnosis of deep head and neck lesions (1, 9-11), and the reported diagnostic accuracy is 87% (1). In addition, no study has specifically addressed the efficacy of CNB in cancer patients, post treatment. Here, we present the results of CT-guided CNB of deep suprahyoid lesions in 28 patients with treated head and neck cancers.
We retrospectively reviewed the clinical records, procedural notes, and histopathologic findings from the 28 consecutive CT-guided CNB procedures in 28 patients (4 female, 24 male, 40-71 years; mean, 54.8 years), with treated head and neck cancers from December, 2003 to May, 2011. Four board-certified neuroradiologists performed the procedures. All record reviews were approved by the research ethics committee board (institutional research board) of our institution.
All target lesions were in the locoregional areas of primary cancer sites in the patients. Before each procedure, diagnostic CT or magnetic resonance imaging (MRI) images were reviewed to assess the anatomical details of the lesions. Biological structures around the lesions were scrutinized to plan an optimal needle pathway that prevented neurovascular injury. Routine blood tests, including international normal ratio, prothrombin time, partial thromboplastin time, hematocrit, and platelet counts were required prior to the biopsy. The necessity and risk of the procedures were explained to each patient and his/her family. Informed consent was obtained prior to each procedure.
The procedures were performed using the GE High Speed Advantage scanner (General Electric Medical Systems, Milwaukee, WI, USA) or the Siemens CT Volume Zoom scanner (Siemens Medical Systems, Erlangen, Germany). Patients were positioned in a supine or lateral decubitus position, depending on the lesion location and the planned needle approach. When necessary, the patient's head was tilted away from the lesion side to facilitate a needle approach. Gantry angulation was adjusted in the axial CT scanning to determine the optimal entry site of the biopsy needle.
To scrutinize the vascular structures in the projected needle path (Fig. 1), intravenous contrast medium was administered in 21 patients (nine lesions in the masticator space, eight in the retropharyngeal space, and two cases in the deep parotid gland and the pterygopalatine fossa, respectively). All procedures were performed under local anesthesia with an appropriate amount of 1% Lidocaine. General anesthesia was not employed.
Biopsies were performed, using the coaxial needle technique, which allows multiple samplings from one percutaneous puncture (12, 13). Needle approaches for lesion access, including subzygomatic, paramaxillary, and retromandibular approaches, were determined by anatomical and technical considerations (1, 3). Using CT imaging guidance, a 17 or 19 gauge introducer needle (10 or 15 cm, respectively; Temno Biopsy Systems, Cardinal Health, Dublin, OH, USA) was inserted into the lesion. An 18 or 20 gauge semi-automatic core biopsy needle (15 cm or 20 cm; Temno Tru-cut Biopsy Systems) was then advanced through the introducer needle to obtain the tissue sample. Two or three tissue cores were typically obtained, depending on the quality of the specimen and the location of the lesion. The obtained specimen was preserved in a standard formalin solution and processed by the pathology department.
In total, 18 patients underwent CNB on an outpatient basis. These patients were observed for two hours after the procedure to ensure their hemodynamic stability. An 18 gauge biopsy needle (with a 17 gauge co-axial needle) was used in 21 procedures, and a 20 gauge needle (with a 19 gauge coaxial needle) was used for the remaining procedures. The number of tissue cores obtained per patient ranged from one to three, with an average of 2.1 needle passes.
Specimen quality and adequacy were assessed by the pathologists. Specimens were considered adequate, if they yielded material sufficient for histological analysis. The final diagnoses were determined by the biopsy results, if performed, and the patient's response to treatment or clinical follow-up. Biopsies, resulting in inadequate specimens or a histologically indeterminate result, were considered incorrect diagnoses. An analysis of the diagnostic accuracy was performed by comparing the biopsy results with the final diagnosis. A Fisher's exact test was used to compare the usage of 18 and 20 gauge needles for a diagnostic yield, diagnostic accuracy, and procedural complication frequency. Patients lost to follow-up were excluded from the analyses.
The clinical profile, details, and results of the 28 biopsy procedures are summarized in Table 1. All patients were clinically followed for 2-63 months, with a mean of 21.6 months. Two patients underwent surgery after the biopsy. The other 26 cases were followed clinically. Lesion dimensions ranged from 2.0 to 4.9 cm, with the mean of 3.2 cm. Lesion depth ranges, measured from the needle entry site on the skin, were from 2.0 to 6.9 cm, with the mean of 3.6 cm. The subzygomatic approach was the most frequent needle approach employed (n = 15), followed by the paramaxillary approach (n = 9).
All obtained specimens were deemed adequate. One specimen taken from a right paraphyaryngeal lesion out of a patient with treated tongue cancer showed atypia (case 27) (Fig. 2). Lesion progression was determined from a one month follow-up MRI study, suggesting a false negative biopsy diagnosis. Among the other 27 adequate specimens, 17 were malignant, and the other 10 were benign. Therefore, the diagnostic accuracy obtained was 27/28 (96.4%).
Minor complications were encountered on two occasions (7.1%). The first was a buccal carcinoma patient, who exhibited a left parapharyngeal mass two years after the surgery and radiotherapy. A biopsy was performed using a 19 gauge coaxial needle and a 20 gauge biopsy needle with a subzygomatic approach. A local hematoma was found in the left messeter muscle, immediately beneath the puncture site (case 20) (Fig. 3). Bleeding was controlled by local compression for 10 minutes. The second case was a patient with suspicious, recurrent cancer in the deep lobe of the left parotid gland. The CNB was performed with a 17 gauge coaxial needle and 18 gauge biopsy needles using a retromandibular approach (case 11) (Fig. 4). Transient facial palsy on the ipsilateral side was noted immediately after the biopsy. The symptoms subsided after 30 minutes of observation. No sequela was noted in either case.
Though statistical comparisons between the use of 18 and 20 gauge needles were made, neither size was more significant than the other, in determining the diagnostic accuracy or limiting procedural complications (p > 0.999 for each gauge).
Biopsies of most head and neck lesions are generally advocated for US guidance. However, US has little usefulness for lesions in the deep and suprahyoid region, due to the limited acoustic window for visualization of the target. CT guidance is well-suited for this type of visualization, as it has less image degradation from the intervening osseous and air-containing structures (5). Though potentially biased by specimen quality, CT-guided FNA is reliable for the head and neck lesions with the diagnostic yield as 90% and accuracy as 86-88% (4, 5). However, in patients with post-treated head and neck cancer, sampling errors can be encountered when small viable tumors are insidious in the treated area. CT-guided CNB presents as an alternative method to obtain tissue for diagnosis of deep head and neck lesions. Generally, CNB can retrieve an adequate, high-quality specimen, and thus, contribute to an accurate diagnosis. However, it is still under investigation whether CNB is more sensitive to detect cancer cells than FNA for head and neck lesions (14).
For head and neck cancer patients, exhibiting new mass lesions in the treated local areas, it is particularly important to differentiate tumor recurrence from a benign process. Needle biopsy plays a crucial role in this clinical setting, as surgery may not be feasible for such patients. To our knowledge, this is the first study reporting CT-guided CNB of deep lesions in post-treated head and neck cancer patients. Our results showed high diagnostic yield (100%) and accuracy (96.4%) of CT-guided CNB for such patients. These findings suggest that an open biopsy can, thus, be avoided. Further, our results were slightly more favorable than those reported in a previous study, describing the core needle biopsy of general skull base lesions (88.9% diagnostic yield and 86.7% accuracy) (1). Our study subjects were patients with post-treated head and neck cancers, which consisted of mucoepithelial carcinoma mostly, while the subjects in the study of Connor and Chaudhary (1) included only four cases with post-treated cancers. Our observed discrepancies may be due to the differences in the study populations and lesion locations.
One false negative result was encountered in our study. A specimen from an infiltrative lesion in the right paraphyaryngeal space displayed atypia (case 27), and tumor progression was confirmed in the follow-up imaging studies. The prevention of such false negative results can be accomplished when encountering a large and infiltrative lesion by targeting the most aggressive part of the lesion, avoiding the necrotic areas, or sampling from multiple sites. In addition, contrast enhancement can also be used for better delineation of the target lesion.
Several needle approach methods have been proposed for the CT-guided needle biopsy of ead and neck lesions (3, 9, 11). The subzygomatic and paramaxillary approaches were used in the majority of our cases. Generally, the subzygomatic approach is suited for the biopsy of lesions in different parts of the masticator space and in the parapharyngeal and retropharyngeal spaces. The paramaxillary approach, using the buccal space as a route, is particularly useful for the lesions in the lateral aspect of retropharyngeal space (11). The transoral approach was not used in any one of our patients because trismus is often a complication in head and neck cancer patients after radiotherapy and operation.
The coaxial technique, with a coaxial needle and a core biopsy needle, has been adapted as an effective approach of CT-guided CNB (1, 3, 9, 11). It allows multiple tissue samplings without additional needle passes. A 17 or 19 gauge coaxial needle, along with an 18 or 20 gauge biopsy needle, respectively, are usually chosen for the biopsy (1, 3, 9, 11). Large-gauge needles generally provide greater diagnostic value as pathologists can more readily determine the specific histological type of mass lesions from the larger tissue core; however the risk of hemorrhage may increase (15). An 18 gauge biopsy needle is widely accepted to be effective and safe in biopsies of the head and neck areas (1, 2), but no previous study has contrasted its use against 20 gauge needles. Our results showed that either an 18 or a 20 gauge biopsy needle yields similar diagnostic outcomes in the biopsy of head and neck lesions. Therefore, determinants in needle gauge choice should be based upon the clinical setting and operators' personal experience.
CT-guided CNB of head and neck lesions has been reported to be a safe technique (1, 3, 16), with major complications occurring rarely. Walker et al. (16) detailed a case with internal maxillary artery pseudoaneurysm formation, three months after CNB in the masticator space. Some minor complications, including pain, vasovagal reaction, minor infection, and minor bleeding, have also been reported (3). Connor and Chaudhary (1) reported no complication in their series for general skull base lesions. Two minor complications were encountered in our study. In the case of the local hematoma in the messeter muscle (Fig. 3), bleeding may have occurred from the inadvertent injury of small vessels during the needle passage. Ten minutes of local compression was able to control the bleeding. Complications like this are rarely reported and are technically limited (3). In the second case, the symptoms of transient nerve palsy were relieved after 30 minutes of observation and no sequela was noted. Cranial nerve injury can theoretically be a concern, but such a risk has not been demonstrated (3). Our results suggest that local inflammation, granulation, and structural alternation, caused by prior surgery and radiotherapy, are likely to increase the risk of these complications; however more evidence is needed to support this hypothesis. Metastasis, along the needle tract, was not observed in our study cohort.
This study was limited by its retrospective nature. Tissue diagnosis confirmation in patients without surgical result can be limited due to varied clinical follow-up schedules between the patients. We were further limited by the number of cases in our study population as the ultrasound-guided biopsy is still the primary diagnostic tool for the approachable head and neck tumors.
In conclusion, CT-guided core needle biopsy is an effective and accurate tool for histological diagnosis of deep suprahyoid lesions in the post-treated head and neck cancer patients, with infrequent minor complications. Diagnostic outcome similarity can be obtained with either an 18 or 20 gauge core biopsy needle. This procedure can offer a definitive tissue diagnosis and avoid unnecessary surgical interventions in patients with head and neck cancers.
Figures and Tables
Fig. 1
Fifty one-year-old male (case 28) with left buccal cancer after surgery and radiotherapy.
A. Contrast-enhanced CT scan shows large necrotic lesion in left masticator space with infiltrative part at periphery (arrow). B. Using subzygomatic approach, 17/18 G biopsy needle set is inserted into lesion (arrow). Biopsy revealed fibrosis with granulation. Follow-up image studies in three and six months showed lesion in stationary status, consistent with biopsy result (not shown).
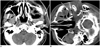
Fig. 2
Sixty four-year-old male (case 27) with history of tongue cancer after operation and radiotherapy.
A. Pre-procedural CT scan shows large infiltrative lesion in right parapharyngeal space (arrow). B. Using retromandibular approach, 17/18 G biopsy needle set is inserted in lesion (arrow). Biopsy revealed atypia. Progressive enlargement of lesion was noted in follow-up CT in one month (not shown). Patient died two months after procedure due to poor condition.
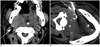
Fig. 3
Sixty four-year-old male (case 20) with buccal cancer after operation and radiotherapy.
A. Contrast-enhanced CT shows infiltrative lesion in left parapharyngeal space (arrow). B. Using subzygomatic approach, 17/18 G biopsy needle set is inserted in lesion. Note hematoma in left messeter muscle (arrow). Biopsy revealed metastatic squamous cell carcinoma.

Fig. 4
Forty-year-old male (case 11) with left parotid adenoid cystic carcinoma after surgical excision.
A. Contrast-enhanced CT shows heterogeneous hypodense lesion in deep lobe of left parotid gland (arrow). B. Using retromandibular approach, 17/18 G needle set is inserted in lesion (arrow). Biopsy revealed adenoid cystic carcinoma. Transient left facial palsy was noted immediately after procedure. Symptom subsided in 30 minutes.

Table 1
Procedural Details, Results, and Follow-Up Courses of 28 Patients Undergoing CT-Guided Core Needle Biopsy

Note.- *Lesion depth from entry point of skin, †No treatment due to poor medical condition. NPC = nasopharyngeal carcinoma, RT = radiotherapy, OP = operation, MS = masticator space, RP = retropharyngeal space, PS = parotid space, PPF = pterygopalatine fossa, SCC = squamous cell carcinoma, ACC = adenoid cystic carcinoma, NKC = non-keratinizing carcinoma, UC = undifferentiated carcinoma, PD = parotidectomy
References
1. Connor SE, Chaudhary N. CT-guided percutaneous core biopsy of deep face and skull-base lesions. Clin Radiol. 2008. 63:986–994.
2. Nyquist GG, Tom WD, Mui S. Automatic core needle biopsy: a diagnostic option for head and neck masses. Arch Otolaryngol Head Neck Surg. 2008. 134:184–189.
3. Gupta S, Henningsen JA, Wallace MJ, Madoff DC, Morello FA Jr, Ahrar K, et al. Percutaneous biopsy of head and neck lesions with CT guidance: various approaches and relevant anatomic and technical considerations. Radiographics. 2007. 27:371–390.
4. DelGaudio JM, Dillard DG, Albritton FD, Hudgins P, Wallace VC, Lewis MM. Computed tomography--guided needle biopsy of head and neck lesions. Arch Otolaryngol Head Neck Surg. 2000. 126:366–370.
5. Sherman PM, Yousem DM, Loevner LA. CT-guided aspirations in the head and neck: assessment of the first 216 cases. AJNR Am J Neuroradiol. 2004. 25:1603–1607.
6. Howlett DC, Harper B, Quante M, Berresford A, Morley M, Grant J, et al. Diagnostic adequacy and accuracy of fine needle aspiration cytology in neck lump assessment: results from a regional cancer network over a one year period. J Laryngol Otol. 2007. 121:571–579.
7. Pfeiffer J, Kayser G, Technau-Ihling K, Boedeker CC, Ridder GJ. Ultrasound-guided core-needle biopsy in the diagnosis of head and neck masses: indications, technique, and results. Head Neck. 2007. 29:1033–1040.
8. Screaton NJ, Berman LH, Grant JW. Head and neck lymphadenopathy: evaluation with US-guided cutting-needle biopsy. Radiology. 2002. 224:75–81.
9. Abrahams JJ. Mandibular sigmoid notch: a window for CT-guided biopsies of lesions in the peripharyngeal and skull base regions. Radiology. 1998. 208:695–699.
10. Mukherji SK, Turetsky D, Tart RP, Mancuso AA. A technique for core biopsies of head and neck masses. AJNR Am J Neuroradiol. 1994. 15:518–520.
11. Tu AS, Geyer CA, Mancall AC, Baker RA. The buccal space: a doorway for percutaneous CT-guided biopsy of the parapharyngeal region. AJNR Am J Neuroradiol. 1998. 19:728–731.
12. Cheung JY, Kim Y, Shim SS, Lim SM. Combined fluoroscopy- and CT-guided transthoracic needle biopsy using a C-arm cone-beam CT system: comparison with fluoroscopy-guided biopsy. Korean J Radiol. 2011. 12:89–96.
13. Tomozawa Y, Inaba Y, Yamaura H, Sato Y, Kato M, Kanamoto T, et al. Clinical value of CT-guided needle biopsy for retroperitoneal lesions. Korean J Radiol. 2011. 12:351–357.
14. Renshaw AA, Pinnar N. Comparison of thyroid fine-needle aspiration and core needle biopsy. Am J Clin Pathol. 2007. 128:370–374.
15. Charboneau JW, Reading CC, Welch TJ. CT and sonographically guided needle biopsy: current techniques and new innovations. AJR Am J Roentgenol. 1990. 154:1–10.
16. Walker AT, Chaloupka JC, Putman CM, Abrahams JJ, Ross DA. Sentinel transoral hemorrhage from a pseudoaneurysm of the internal maxillary artery: a complication of CT-guided biopsy of the masticator space. AJNR Am J Neuroradiol. 1996. 17:377–381.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download