Abstract
Osseous hemangioma is a benign vascular tumor, and it usually occurs in the vertebrae and the skull. However, hemangiomas of flat bones are rare, and there are very few reports that describe the radiologic findings of osseous hemangioma of the ilium. We report a unique case of large cavernous hemangioma mimicking a chondrogenic malignant bone tumor originated from the ilium in a 22-year-old female. The mass showed stippled calcifications, heterogeneous enhancement with thick septa and enhanced soft tissue components on CT and MR, and also this mass demonstrated heterogeneous 2-fluoro [fluorine-18]-2-deoxy-D-glucose (18F-FDG) uptake on 18F-FDG PET/CT.
Osseous hemangioma is a benign vascular tumor and most frequently involves the bones of the axial skeleton (1, 2). Osseous hemangiomas in the peripheral, tubular and flat bones are uncommon, and very few reports have described the radiologic findings of the osseous hemangioma of the ilium (1-3). Osseous hemangiomas may have a wide variety of radiologic appearances, and the lesions occurred in the tubular or flat bones can be demonstrated as aggressive tumors showing cortical destruction with extraosseous mass formation and osteolytic lesions mimicking a malignant bone tumor (1-6). On 2-fluoro [fluorine-18]-2-deoxy-D-glucose (18F-FDG) positron emission tomography (PET), hemangioma has been classified as a metabolically stable tumor with low 18F-FDG uptake, but a recently reported case of hemangioma of the tibia showed high 18F-FDG uptake on PET/CT (7).
We present a case of a cavernous hemangioma of the ilium mimicking an aggressive malignant bone tumor, which demonstrated a large osteolytic lesion with cortical destruction, extraosseous soft tissue mass formation, and irregular calcifications on CT and MR. In addition, we describe the 18F-FDG PET/CT findings of the osseous hemangioma of the ilium, which showed heterogeneous 18F-FDG uptake.
A 22-year-old female presented with a gradually increasing pelvic mass with tenderness for 1 month. There was no evidence of previous trauma or fever, and also there was no history of other medical or surgical disease and no evidence of pregnancy. On the physical examination, there was a palpable mass with tenderness in her left pelvic area. A plain radiograph of the pelvis showed a huge, geographic osteolytic lesion with a thick and irregular sclerotic rim in the left ilium (Fig. 1A). CT showed an ill marginated, 13 × 10 cm, osteolytic lesion of the left ilium with extraosseous soft tissue mass formation and intralesional stippled calcifications. On the contrast-enhanced CT, the mass showed irregular, rim enhancement and focal enhanced soft tissue components (Fig. 1B, C). On MRI, the lesion had a heterogeneous intermediate signal on the T1-weighted images, a heterogeneous high signal on fat suppressed T2-weighted images, and irregular low signal septa on T1 and T2-weighted images. After enhancement with gadolinium, the mass was demonstrated as a heterogeneously enhanced mass, as well as non-enhanced soft tissue components and septal structures (Fig. 1D-F). On 99mTc hydroxymethane diphosphonate (HDP) bone scintigraphy, increased isotope uptake was depicted in the left iliac bone (Fig. 1G). After fasting for 6 hours, intravenous injection of 375 MBq of 18F-FDG was performed (at that time, her blood glucose level was 92 mg/dL). The PET scan (3 min/bed, 6-8 beds) was obtained from 60 minutes post 18F-FDG injection. 18F-FDG PET/CT scanning was done by Discovery PET/CT scanner (GE Healthcare, Milwaukee, WI, USA), and scans were acquired in 3D mode. Reconstruction was done by ordered subset expectation maximization (OSEM) (128 × 128 matrix, 3.27 mm slice thickness, subsets: 21, iteration: 2, post-Filter: 5.14 FWHM (mm), loop-Filter: 4.69 FWHM, diameter: 60 cm) after attenuation correction. 18F-FDG PET/CT demonstrated heterogeneous 18F-FDG uptakes, which had low and high 18F-FDG uptake (maximum standard uptake value [SUVmax] = 5.1) within the mass (Fig. 1H-J). Based on the imaging features, chondrogenic malignant bone tumor in the ilium was considered even though, when taking her age into account, it was unusual. Complete removal of the tumor was performed. On gross examination, the mass appeared as a vascular mass containing central necrosis, irregular bony septa, and fibrous tissues. Histopathologic examination of the excised mass showed bony trabeculae with dilated vascular spaces filled with blood and endothelial lining (Fig. 1K), and it was confirmed as cavernous hemangioma of the ilium by the experienced musculoskeletal pathologists.
Intraosseoous hemangiomas are benign hamartomatous vascular tumors of the bones, and they frequently involve the skull and vertebral column (1-3). The peripheral, long, tubular and flat bones are the least affected sites, and among them, the tibia and femur are the most common sites (1). Very few cases of hemangioma affecting the pelvic bones have been reported (1-3). Hemangiomas involving the vertebrae or skull are usually asymptomatic and they are often found incidentally on routine imaging studies. However, hemangiomas in the peripheral and pelvic bones are usually symptomatically associated with pain, soft tissue swelling and pathologic fracture (1, 2, 5). Our patient complained of tenderness with a palpable mass in her pelvis for a duration of approximately one-month.
Intraosseous hemangiomas may have various radiologic findings. Due to the diversity of radiologic features of this disease, a correct preoperative diagnosis is rarely made. On plain radiographs, osseous hemangiomas may show a loculated, sunburst, moth-eaten, and soap bubble appearance due to engorged vessels and thickened trabeculae, and they rarely manifest expansile osteolytic changes (1-8). On CT, hemangiomas in the skull and spine can present a typical polka-dot or honeycomb appearance; however CT findings of the hemangiomas in long and flat bones are non-specific and it is difficult to take into consideration osseous hemangiomas in a differential diagnosis. They can be demonstrated as expanded lytic areas surrounded by coarse trabecular bone with or without a soft tissue component, a lesion with a "polka-dot" pattern, an osteoplastic lesion with a sun-burst-like pattern, honeycombed appearance with cortical defects, an irregular destructive lesion with scalloping on the inner surface of the sclerotic cortical bone, and occasionally calcifications have been reported (1-4, 6, 8, 9). In our case, the mass was depicted as an osteolytic lesion with cortical destruction and extraosseous soft tissue mass formation, stippled calcifications on CT, and enhanced, exophytic soft tissue components on contrast-enhanced CT mimicking chondrogenic malignant bone tumors.
On MR, osseous hemangiomas usually show a variety of signals from low to very high signal intensities on T1- and T2-weighted images within the lesion according to the internal components such as dilated vessels, hemorrhage, blood clots, fibrosis, bony trabeculations, fat, and smooth muscles (1-7). Osseous hemangiomas can show variable degrees of enhancement related to internal contents, as we mentioned above, after contrast administration. Low signal septal structures within the lesion on T1 and T2-weighted images, and the appearance of a bunch of grapes on T2-weighted and Gd-enhanced images were also reported (3, 6-8). In our case, non-enhanced, thick, irregular, septa like structures of low signal intensity within the mass were demonstrated on MR, and we retrospectively thought that this finding could be perceived as a soap-bubble appearance lesion that can be shown in the usual osseous hemangiomas.
18F-FDG PET/CT is increasingly being used to obtain more information for differentiating benign and malignant tumors. Even though, there are some false positive and false negative 18F-FDG uptakes in benign and malignant bone tumors, respectively, PET is generally used in the diagnostic work-up of various musculoskeletal tumors. Hemangioma has been known to be a metabolically stable benign lesion on PET. In vertebral hemangioma, it has been reported to demonstrate from ametabolic, cold vertebra to low 18F-FDG uptake on PET/CT images. This finding can help to differentiate a hemangioma from a metastatic lesion in the case of a vertebral hemangioma depicted as a photopenic defect at the 99mTc MDP bone scan, which need to be differentiated from osteolytic metastatic lesions in patients with a malignant disease. (10, 11). Hatayama et al. (12) have described the SUVs for 18F-FDG in 16 hemangiomas as ranging from 0.73 to 1.67, and Tian et al. (13) have described the SUVs for 18F-FDG in 4 hemangiomas as ranging from 1.6 to 2.3. However, Cha et al. (7) recently reported the case of an intra-osseous hemangioma of the tibia which showed increased 18F-FDG uptake (SUV = 3.9) on PET/CT. Similar to the case reported from Cha et al. (7), our case of the osseous hemangioma of the ilium showed heterogeneously increased 18F-FDG uptake (SUVmax = 5.1) on PET/CT. Sakurai et al. (14), and Higashiyama et al. (15) had described that 18F-FDG PET may provide useful information to differentiate hemangioma from malignant soft tissue tumors owing to the tendency of low 18F-FDG uptake in soft tissue hemangiomas. However, contrary to soft tissue and vertebral hemangiomas, osseous hemangiomas in the peripheral and flat bones can show high 18F-FDG uptake on PET/CT as in our case and the case from Cha et al. (7). To our knowledge, there was no radiologic report describing osseous hemangiomas of the ilium which demonstrated an increased 18F-FDG uptake on PET/CT.
Intraosseous hemangiomas of the long bones and ribs can be rarely demonstrated as an aggressive lesion showing extraosseous growth with the disrupted bony cortex (1-4, 8). The differential diagnosis for a hemangioma of long bones included a giant cell tumor, aneurysmal bone cyst, fibrous dysplasia, and plasmacytoma (1, 5). In the case of hemangiomas of the flat bones which show aggressive growth and osteolytic lesions, malignant bone tumors can be considered as a primary differential diagnosis (1, 3-5). In our case, chondrosarcoma was the primary differential diagnosis although the incidence of the chondrosarcoma is rare for her age.
In conclusion, cavernous hemangioma of the ilium can show cortical destruction, stippled calcifications, and an extraosseous soft tissue mass formation, which can give the false impression of a chondrosarcoma. Furthermore, osseous hemangiomas in the peripheral and flat bones can demonstrate high 18F-FDG uptake on PET/CT. Although osseous hemangioma of the ilium is rare, when a young patient presented with an osteolytic lesion with an extraosseous soft tissue mass, stippled calcifications and thick septa or coarse traculations, osseous hemangioma should be taken into consideration in the differential diagnosis. However, the final diagnosis can only be confirmed by histopathologic examination.
Figures and Tables
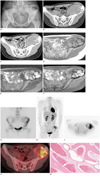 | Fig. 1Cavernous hemangioma of ileum in 22-year-old female mimicking aggressive malignant bone tumor with increased activity on 18F-FDG PET/CT.
A. Plain radiograph of pelvis shows huge, geographic osteolytic lesion with thick, irregular sclerotic rim in left ilium. B, C. On precontrast CT image (B), there is irregular, large osteolytic lesion in left ilium with exophytic soft tissue mass and stippled calcifications. On contrast-enhanced CT (C), mass shows heterogeneous enhancement with irregular, rim and septal enhancement. D-F. On MRI, mass has heterogeneous intermediate signal on T1-weighted image (D), heterogeneous high signal on fat suppressed T2-weighted image (E), and thick irregular low signal septa on T1 and T2-weighted images. After enhancement with gadolinium, mass shows heterogeneous enhancement and non-enhanced septa (F). 18F-FDG = 2-fluoro [fluroine-18]-2-deoxy-D-glucose. G. 99mTc hydroxymethane diphosphonate bone scan. Anterior view shows increased peripheral isotope uptake of left iliac bone lesion. H-J. 18F-FDG PET MIP (H), PET axial (I), and PET/CT fusion axial (J) images show heterogeneously increased FDG uptake of left iliac bone lesion (maximum SUV = 5.1). K. Photomicrograph shows dilated blood-filled vessels lined by flattened endothelial cells (H/E, × 200).
|
References
1. Kaleem Z, Kyriakos M, Totty WG. Solitary skeletal hemangioma of the extremities. Skeletal Radiol. 2000. 29:502–513.
2. Ogose A, Hotta T, Morita T, Takizawa T, Ohsawa H, Hirata Y. Solitary osseous hemangioma outside the spinal and craniofacial bones. Arch Orthop Trauma Surg. 2000. 120:262–266.
3. Puig J, Garcia-Pena P, Enriquez G, Huguet P, Lucaya J. Intraosseous haemangioma of the ilium. Pediatr Radiol. 2006. 36:54–56.
4. Tew K, Constantine S, Lew WY. Intraosseous hemangioma of the rib mimicking an aggressive chest wall tumor. Diagn Interv Radiol. 2011. 17:118–121.
5. Chawla A, Singrakhia M, Maheshwari M, Modi N, Parmar H. Intraosseous haemangioma of the proximal femur: imaging findings. Br J Radiol. 2006. 79:e64–e66.
6. Yamamoto T, Kurosaka M, Mizuno K. Juxta-articular hemangioma of long bone. Skeletal Radiol. 2000. 29:535–537.
7. Cha JG, Yoo JH, Kim HK, Park JM, Paik SH, Park SJ. PET/CT and MRI of intra-osseous haemangioma of the tibia. Br J Radiol. 2012. 85:e94–e98.
8. Matsumoto K, Ishizawa M, Okabe H, Taniguchi I. Hemangioma of bone arising in the ulna: imaging findings with emphasis on MR. Skeletal Radiol. 2000. 29:231–234.
9. López-Barea F, Hardisson D, Rodríguez-Peralto JL, Sánchez-Herrera S, Lamas M. Intracortical hemangioma of bone. Report of two cases and review of the literature. J Bone Joint Surg Am. 1998. 80:1673–1678.
10. Domínguez M, Rayo J, Serrano J, Sánchez R, Infante JR, García L, et al. Vertebral hemangioma: "Cold" vertebrae on bone scintigraphy and fluordeoxy-glucose positron emission tomography-computed tomography. Indian J Nucl Med. 2011. 26:49–51.
11. Bybel B, Raja S. Vertebral hemangiomas on FDG PET scan. Clin Nucl Med. 2003. 28:522–523.
12. Hatayama K, Watanabe H, Ahmed AR, Yanagawa T, Shinozaki T, Oriuchi N, et al. Evaluation of hemangioma by positron emission tomography: role in a multimodality approach. J Comput Assist Tomogr. 2003. 27:70–77.
13. Tian R, Su M, Tian Y, Li F, Li L, Kuang A, et al. Dual-time point PET/CT with F-18 FDG for the differentiation of malignant and benign bone lesions. Skeletal Radiol. 2009. 38:451–458.
14. Sakurai K, Hara M, Ozawa Y, Nakagawa M, Shibamoto Y. Thoracic hemangiomas: imaging via CT, MR, and PET along with pathologic correlation. J Thorac Imaging. 2008. 23:114–120.
15. Higashiyama S, Kawabe J, Hayashi T, Kurooka H, Oe A, Kotani J, et al. A case of cavernous hemangioma in which malignancy was preoperatively excluded by FDG-PET. Ann Nucl Med. 2008. 22:327–330.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download