Abstract
A pulmonary artery aneurysm is a common manifestation and the leading cause of mortality in Behçet's disease. We describe a case of spontaneous rupture of a pulmonary artery aneurysm that, due to the inadequacy of medical therapy and the disadvantages of surgery, became the ideal candidate for endovascular management and was successfully performed by using the Amplatzer Vascular Plug 4.
Behçet's disease (BD) is a multisystem disorder characterized by recurrent symptoms and vasculitis. The triple symptom complex of oral and genital ulcerations and uveitis reported by Hippocrates and other authors was originally attributed to major infections. However, the Turkish dermatologist Hulusi Behçet discarded the association with other illnesses and was the first to delineate the disease that now bears his name (1). The syndrome is more frequent in men than in women (male to female ratio is almost two to one) and is most prevalent in the Mediterranean region, Middle East, and Far East, with the highest prevalence in Turkey. The cause of BD is unclear, but genetic predisposition (human leukocyte antigen-B51) and certain microorganisms are believed to play a role (1).
According to the diagnostic criteria of the International Study Group of BD (1), the diagnosis is made on the detection of oral ulcers and at least two of the following criteria: recurrent genital ulcers, ocular lesions including uveitis and retinal vasculitis, skin lesions and positive skin pathergy test (pustule formation 24-48 hours after skin prick).
Other organs affected by BD include the central nervous system, gastrointestinal tract and respiratory system.
To our knowledge there are few reports concerning the emergency endovascular management of pulmonary artery aneurysms (PAA) in BD, but in none of these was the Amplatzer Vascular Plug (AVP-AGA Medical Corp., Plymouth, MN, USA) used as an embolic agent.
A 37-year-old man was admitted to our hospital with severe hemoptysis, and diagnosed with BD 5 years ago at another hospital. A small PAA had been treated by endovascular coil embolization and discharged with medical treatment consisting of prednisolone and cyclophosphamide which he had taken for only a few months.
The patient did not complain of fever or chills. In addition, his hemoglobin level was 11.1 g/dL and hematocrit was 35.3%. Pulse oximetry showed a moderate desaturation to 86% on room air.
Chest radiography showed an alveolar-type opacity in the left upper lobe. Because of worsening hemoptysis, urgent thoracic computed tomography (CT) scanning was performed before and after administration of 100 mL nonionic contrast material (Iomeron 350; Bracco, Milano, Italy) injected at 3.5 mL/sec through a 19-gauge peripheral intravenous catheter in the antecubital vein, with axial scanning from the lung apices to the diaphragm with a 4-section CT scanner (Somatom Sensation; Siemens Medical Solutions, Erlangen, Germany) (Fig. 1A). CT images revealed a 27 × 19 mm aneurysm in the left upper lobe originating from the posterior segmental artery (Boyden A3), which was surrounded by an area of ground-glass opacity suggestive of vasculitis and hemorrhage (Fig. 1A, B).
Written informed consent for embolization was obtained from the patient. After placement of a 5 Fr introducer sheath (Cordis, Miami, FL, USA) in the right femoral vein under local anesthesia, a diagnostic pulmonary angiography (Axiom Artis; Siemens Medical Solutions, Erlangen, Germany) confirmed the PAA in the left upper lobe (Fig. 1C). The posterior segmental artery was approximately 5 mm in diameter at the aneurysm neck. A 4 Fr Cobra catheter (Tempo Aqua; Cordis Corporation, Miami, FL, USA) was introduced into the posterior segmental artery with the help of digital roadmapping and a 0.035-inch, 180-cm angled guidewire (Radifocus; Terumo, Tokyo, Japan). The PAA was embolized with an 8-mm AVP 4 (Fig. 1D). Repeat angiography after embolization confirmed the occlusion of the aneurysm (Fig. 1D). The patient did not have any further hemoptysis in the immediate post-embolization period, and his oxygen saturation progressively increased.
Chest radiography on day 3 after the embolization showed partial resolution of the left upper lobe alveolar-type opacity (Fig. 1E), and the patient was discharged on day 4 with antibiotic prophylaxis and an immunosuppressive regimen.
Behçet's disease is a progressive systemic disorder characterized by exacerbations and remissions of unpredictable duration. As there are no pathognomonic symptoms or laboratory findings, BD is diagnosed using the diagnostic criteria defined by the International Study Group for BD (1-3).
Vasculitis caused by BD can occur in three forms: 1) venous occlusion and varix formation, 2) arterial occlusion and/or pulseless disease and 3) arterial aneurysm formation (3, 4): such form of BD is called "vasculo-BD" (5). Pathologic findings include intense inflammatory cell infiltration, especially around the vasa vasorum, as well as severe destruction of the media and disarray of the internal elastic membrane during the active stage of the disease and periadventitial fibrosis at the chronic stage of the disease (3). Arterial involvement is less common than venous involvement and accounts for 12% of vascular manifestations. Arterial lesions may develop in the aorta or in the pulmonary arteries and their major branches, occurring as an aneurysm in 65% of patients and as an occlusion in 35% (5).
Behçet's disease is the leading cause of PAAs (5). The first case of BD-associated pulmonary problems was published in 1959 (6), and many other cases with various pulmonary problems have since been reported. The reported prevalence of pulmonary problems in patients with BD varies widely, from < 1% to 18%. The main features of pulmonary involvement are PAAs, arterial and venous thrombosis, pulmonary infarction, recurrent pneumonia, bronchiolitis obliterans, organised pneumonia and pleurisy (1). Pulmonary disorders are among the most common direct causes of death in BD. The 1- and 5-year survival rates of BD patients with PAAs are 57% and 39%, respectively (6).
Pulmonary artery aneurysms occur mainly in young men. Hemoptysis of varying degrees (up to 500 mL) is the most common and predominant symptom, and is thought to be due to either rupture of an aneurysm with erosion into a bronchus or the development of in situ thrombosis secondary to active vasculitis (6). PAAs are most frequently located in the arteries of the right lower lobe, followed by the right and left main pulmonary arteries. In most patients, PAAs are multiple, bilateral, sacculated, and partially or completely thrombosed (6).
A variety of treatment modalities, such as immunosuppression, surgery, anticoagulation and embolization, have been used in the management of PAAs in BD.
Immunosuppressive drug therapy alone or in combination with steroids is most beneficial when given administered during early stages of the disease, before the development of irreversible damage to the arterial wall. Tunaci et al. (7) reported the complete disappearance or regression of PAAs during a follow-up period of 3-42 months (mean, 21 months) in 13 patients receiving immunosuppressant treatment. In our patient, life-threatening hemoptysis required more rapid treatment that can't be achieved using medical therapy.
Because of the nature of the disease, emergency surgery carries a high risk of complications such as perivascular leaks, graft thrombosis, and anastomotic leaks (3); recurrence of hemoptysis has also been reported in some patients (2). Aneurysmorrhaphy, lobectomy, bilobectomy, pleurectomy, aneurysmectomy, and pneumonectomy have all been used in the management of BD patients with PAAs (6). Surgical therapy has the disadvantage of the potential need for repeated thoracotomy because of recurrent aneurysm formation. Postoperative healing may be compromised because most patients are taking long-term corticosteroids, which also increased the risk of infection (2, 6). As PAAs commonly occur bilaterally, increased pulmonary artery pressure after lobectomy may result in increased size of other PAAs with subsequent rupture and death (6). Because of these disadvantages and the high mortality rate of surgical therapy, endovascular treatment may be a reasonable alternative to surgery in the presence of life-threatening hemoptysis.
It was recently reported that patients treated by embolization with or without immunosuppressive therapy had a better prognosis than patients who underwent surgery with or without immunosuppressive therapy (6). A review of the literature identified two embolic agents which have previously been used to treat PAAs: coils and n-butyl cyanoacrylate (NBCA) (2, 3, 8-10). Metallic coils, which are simple but are expensive if many are required (2), are the preferred option for permanent occlusion of arterial aneurysms (5). As PAAs are pseudoaneurysms (1) with fragile walls, the use of coils may have disastrous consequences if there is periprocedural rupture of the PAA. However, the risk of aneurysm rupture remains theoretical as there have not been any such reported cases.
N-butyl cyanoacrylate is widely used as a liquid embolic material to treat brain and peripheral arteriovenous malformations, and has also been used for the embolization of tumors, gastrointestinal bleeding, the deep dorsal vein of the penis to treat impotence caused by veno-occlusive dysfunction, varicoceles, and endoleaks (10). There have been some reports of successful use of NBCA, also with the "bubble technique" (3) for the treatment of PAAs (2, 8). This embolic agent has been proven to be safe in experienced hands, but carries the potential risks of catheter adhesion or distal migration of the glue. Although the occlusion created by the glue may be permanent, recanalization has occurred in some cases, especially when only partial embolization was achieved (10).
The latest generation of AVP (AVP 4) is a self-expanding device made of two fine mesh lobes of nitinol wire, and is available in 4-8 mm diameter sizes, in 1-mm increments. Platinum marker bands at both ends of the device make it highly visible under fluoroscopy. The AVP 4 has a microscrew attachment connected to a 155-cm-long, PTFE-coated delivery wire.
The main advantage of the AVP, compared with other embolization devices such as metallic coils, is that it can occlude the feeding and/or draining vessels of an aneurysm or arteriovenous malformation with a single device, thus having more rapid or immediate results. Another advantage is that the delivery system enables increased precision and control during deployment (11). The elasticity of nitinol allows the device to become firmly anchored to the vessel wall by outward radial force, which prevents migration and allows lengthening to be predicted. As a result, selection of the diameter of the AVP does not need to be as precise as selection of the correct diameter, length, and type when using a coil. Hence, the AVP offers an alternative to occlusion with metallic coil embolization, which requires the placement of several coils of different sizes with 4 Fr or 5 Fr catheters or the more expensive microcatheters to occlude a single vessel (11). The AVP also causes fewer metallic artifacts on CT angiography than platinum coils, because of its morphological and structural characteristics (11). Finally, a test injection of contrast medium through the delivery catheter can verify the position of the device before release, and the device can easily be repositioned if the initial deployment position is not desirable (11). Compared with the previous generation of vascular plug, the main advantage of the fourth generation plug is that it can be placed using a diagnostic catheter as small as 4 Fr, which is flexible enough to negotiate tortuous vessels or acute angles and therefore enables more peripheral placement of the device (11).
Figures and Tables
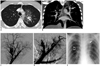
Fig. 1
Ruptured pulmonary artery aneurysm treated with Ampltazer Vascular Plug 4.
A. Axial contrast-enhanced CT scan (lung window) shows aneurysm of left upper lobe pulmonary artery (arrow) accompanied by mural thrombus and surrounded by diffuse ground-glass opacity at upper left pulmonary lobe, which is suggestive of hemorrhage. B. Coronal maximum-intensity-projection CT image depicts left pulmonary artery aneurysms at level of upper lobe (arrow). C. Left pulmonary angiography via right femoral vein on anteroposterior (AP) projection demonstrates aneurysm at upper lobe pulmonary artery. D. Post-embolization pulmonary angiography on AP projection shows Amplatzer Vascular Plug 4 (AVP 4) with no further filling of aneurysmal sac. E. Chest radiograph demonstrates partial resolution of left upper lobe alveolar-type opacity (still recognizable), presence of AVP 4 (arrow), and coils (arrowheads) due to previous treatment.
References
1. Ceylan N, Bayraktaroglu S, Erturk SM, Savas R, Alper H. Pulmonary and vascular manifestations of Behcet disease: imaging findings. AJR Am J Roentgenol. 2010. 194:W158–W164.
2. Cantasdemir M, Kantarci F, Mihmanli I, Akman C, Numan F, Islak C, et al. Emergency endovascular management of pulmonary artery aneurysms in Behçet's disease: report of two cases and a review of the literature. Cardiovasc Intervent Radiol. 2002. 25:533–537.
3. Cil BE, Geyik S, Akmangit I, Cekirge S, Besbas N, Balkanci F. Embolization of a giant pulmonary artery aneurysm from Behcet disease with use of cyanoacrylate and the "bubble technique". J Vasc Interv Radiol. 2005. 16:1545–1549.
4. Park JH, Han MC, Bettmann MA. Arterial manifestations of Behçet disease. AJR Am J Roentgenol. 1984. 143:821–825.
5. Cil BE, Turkbey B, Canyiğit M, Kumbasar OO, Celik G, Demirkazik FB. Transformation of a ruptured giant pulmonary artery aneurysm into an air cavity after transcatheter embolization in a Behçet's patient. Cardiovasc Intervent Radiol. 2006. 29:151–154.
6. Uzun O, Erkan L, Akpolat I, Findik S, Atici AG, Akpolat T. Pulmonary involvement in Behçet's disease. Respiration. 2008. 75:310–321.
7. Tunaci M, Ozkorkmaz B, Tunaci A, Gül A, Engin G, Acunaş B. CT findings of pulmonary artery aneurysms during treatment for Behçet's disease. AJR Am J Roentgenol. 1999. 172:729–733.
8. Lacombe P, Frija G, Parlier H, Lang F, Hamza M, Hamza R, et al. Transcatheter embolization of multiple pulmonary artery aneurysms in Behçet's syndrome. Report of a case. Acta Radiol Diagn (Stockh). 1985. 26:251–253.
9. Lacombe P, Qanadli SD, Jondeau G, Barré O, Mesurolle B, Mouas H, et al. Treatment of hemoptysis in Behçet syndrome with pulmonary and bronchial embolization. J Vasc Interv Radiol. 1997. 8:1043–1047.
10. Pollak JS, White RI Jr. The use of cyanoacrylate adhesives in peripheral embolization. J Vasc Interv Radiol. 2001. 12:907–913.
11. Laganà D, Carrafiello G, Mangini M, Lumia D, Fontana F, Ianniello A, et al. Indications for the use of the Amplatzer vascular plug in interventional radiology. Radiol Med. 2008. 113:707–718.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download