Abstract
Objective
To evaluate the therapeutic efficacy and safety of percutaneous ethanol injection (PEI) alone and combined with radiofrequency ablation (RFA) for hepatocellular carcinomas (HCCs) in high risk locations.
Materials and Methods
We performed PEI for HCCs in RFA-high risk locations, either alone or in combination with RFA. There were 20 HCCs (1.7 ± 0.9 cm) in 20 patients (PEI group: n = 12; PEI + RFA group: n = 8). We evaluated technical success, local tumor progression and complications in both groups.
Results
Technical success was achieved in all HCCs in both groups. During follow-up, local tumor progression was found in 41.7% (5/12) in the PEI group, whereas 12.5% (1/8) for the PEI + RFA group (p = 0.32). Bile duct dilatation was the most common complication, especially when the tumors were in periportal locations; 55% (5/9) in the PEI group and 50% (2/4) in the PEI + RFA group (p = 1.00). One patient in the PEI group developed severe biliary stricture and upstream dilatation that resulted in atrophy of the left hepatic lobe. One patient treated with PEI + RFA developed cholangitis and an abscess.
Conclusion
Combined PEI and RFA treatment has a tendency to be more effective than PEI alone for managing HCCs in high risk locations, although the difference is not statistically significant. Even though PEI is generally accepted as a safe procedure, it may cause major biliary complications for managing HCCs adjacent to the portal vein.
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world, and its incidence is increasing worldwide (1-4). Although surgical resection may provide the opportunity for a complete cure, its role is limited due to poor liver function of HCC patients, as well as multiplicity of tumors. Therefore, non-surgical locoregional therapies, such as radiofrequency ablation (RFA) and percutaneous ethanol injection (PEI), have been used worldwide. There have been several randomized controlled trials which have compared the outcomes of these two procedures and all demonstrated that RFA showed lower local tumor progression rates and higher survival rates (5, 6). This is mainly because the spread of ethanol is affected by the capsule or septa of HCC lesions and therefore the effect of PEI is less predictable, whereas in RFA, the heat generated around the electrode tip distributes homogeneously in all directions and therefore the ablated zone is more predictable, resulting in better technical success and improved prognosis (7, 8). For these reasons, PEI has been widely replaced by RFA.
However, the efficacy of RFA can vary depending on the location of HCC lesions. HCC lesions in close proximity to various structures, such as blood vessels, central bile ducts and extrahepatic vital organs, are considered to be at high risk of treatment failure and complications due to their location (4, 9-11). In these cases, the efficacy of RFA is lower, mainly due to less optimal positioning of the RFA electrode to avoid thermal injury to these various structures. Also, the heat-sink effect produces smaller areas of coagulation necrosis and therefore lowers the technical success rates. In these cases, PEI has its role when used either alone or in combination with RFA, according to the size or location of the tumors (11, 12). Even though PEI may be used in treating HCC lesions in RFA-high risk locations, there have been few studies focused on its therapeutic efficacy and safety. Therefore, the purpose of this study was to evaluate the therapeutic efficacy and safety of PEI alone and combined PEI with RFA for HCC lesions in high risk locations.
This retrospective study was approved by our institutional review board and written informed consents were waived. We conducted a retrospective review of our database of patients who underwent PEI, with or without RFA, as a local ablation therapy for HCCs, between February 2009 and June 2010. In our institution, RFA is considered as the primary treatment modality for local ablation therapy of HCC, because RFA is superior to PEI in terms of local tumor control. Inclusion criteria for percutaneous RFA in our institution are as follows: a single tumor ≤ 4 cm in the longest diameter, multinodular tumors (n ≤ 3) with each tumor ≤ 3 cm in the longest diameter, Child-Pugh class A or B, no portal vein thrombosis or extrahepatic metastasis, a prothrombin time ratio of > 50%, and a platelet count > 50000 cells/mm3 (50 cells × 109/L). However, when the index tumor was in the so-called "high risk location", HCC tumors have been treated with PEI alone or in combination with RFA. We defined 'high risk locations' when the index tumor was a perivascular lesion, which means the tumor was in contact with vessels greater than or equal to 3 mm in diameter (9), or when the index tumor was a subcapsular lesion, thus in contact with the gallbladder, colon, or duodenum. In terms of tumors contacting the gastrointestinal tract, if the tumor was separated from the gastrointestinal tract using artificial ascites, we performed RFA, rather than PEI. All patients with tumors contacting the gastrointestinal tract who enrolled in this study had a history of laparotomy and resultant intra-abdominal adhesions by which the gastrointestinal tract could not be separated from the index tumor, even after the use of artificial ascites. Therefore, in order to avoid collateral thermal injury, we managed these tumors with PEI, rather than RFA.
Generally, if the size of the index tumor in the high risk location was smaller than 2 cm in diameter, we performed PEI only without RFA, since PEI is known to be as effective as RFA for HCCs smaller than 2 cm (6). However, if the size of the tumor in the high risk location was larger than 2 cm, we performed PEI in combination with RFA. PEI was performed on the part of the tumor closest to the adjacent 'dangerous' structures that needed attention during ablation procedures, whereas RFA was performed on the part of tumor farthest from these adjacent structures (11). In addition, even though the size of the tumor was smaller than 2 cm, when the echogenic zone induced by PEI was not large enough to cover the entire tumor and the residual non-ablated area was apart from the dangerous adjacent structures, we additionally performed the RFA procedure to these residual areas. In these cases, we placed the RFA electrode tip more than 1.5 cm apart from the adjacent dangerous structures.
A total of 23 consecutive patients who underwent PEI for HCC were found using a computer-aided search. Among them, 3 patients were excluded due to liver transplantation (n = 1) during the follow-up period and loss to follow-up (n = 2). Therefore, 20 patients (13 men and 7 women; age range, 47-84 years; mean age, 59.1 ± 9.5 years) were evaluated in this study. All 20 patients had cirrhosis of the liver associated with viral hepatitis B (n = 15), hepatitis C (n = 3), both hepatitis B and C (n = 1), or unknown etiology (n = 1). According to the Child-Pugh classification, all patients were classified as class A. Five patients had no treatment history of HCC whereas the other 15 patients had previous treatment history of HCC, by the following methods: hepatic resection (n = 3), RFA (n = 4), transcatheter arterial chemoembolization (TACE) (n = 4), hepatic resection + TACE (n = 1), and TACE + RFA (n = 3). These 20 patients had 24 HCCs: single HCC in 16 patients and two HCCs in 4 patients. For each of the 4 patients who had two HCCs, only tumors in high risk locations and treated with PEI were included in this study. The other four tumors located in non-high risk locations and treated with RFA (mean size ± SD, 2.0 ± 1.0 cm) were not of interest in this study and thus excluded. Therefore, 20 HCCs (mean size ± standard deviation [SD], 1.7 ± 0.9 cm; range, 1.0-5.2 cm) in 20 patients were evaluated. Thirteen HCCs were newly detected lesions and had no previous treatment history, whereas the other 7 lesions were recurrent HCC after TACE (n = 6) or RFA (n = 1). The locations of the 20 HCCs were, as follows: hepatic segment 3 (n = 1), 4 (n = 2), 5 (n = 5), 6 (n = 2), 7 (n = 1) and 8 (n = 9). The mean time interval between the last CT and percutaneous PEI was 8.9 ± 6.4 days (range, 0-30 days).
The diagnosis of HCC was based on the typical imaging features (arterial enhancement followed by delayed washout) on the dynamic contrast-enhanced CT and/or MRI images (13). Percutaneous biopsy was not performed in any patient.
All ablation procedures were performed on an inpatient basis by one of three radiologists. They were all board-certified, experienced abdominal radiologists who each had more than 4 years of experience and had performed more than 200 RFA or PEI procedures prior to starting this study. For local anesthesia, we injected 2% lidocaine hydrochloride (Huons Lidocaine HCI INJ; Hwaseong, Korea) at the puncture site. In addition, for pain control, 50 mg of pethidine hydrochloride (Pethidine, Samsung Pharmaceutical, Seoul, Korea) mixed with 50 mL of 5% dextrose in water was dripped continuously by intravenous infusion. If needed, 100 µg of fentanyl citrate (Fentanyl Citrate Gu Ju INJ; GUJU Pharma, Seoul, Korea) mixed with 20 mL of normal saline was divided into 4 even-quarters and was injected intermittently during the RFA procedure. After local anesthesia, one or two 21-gauge PEI needles with six side holes (PEIT; Hakko, Tokyo, Japan) were inserted in the lower edge of the tumor under ultrasound guidance with a 4-1-MHz convex probe (Acuson Sequoia 512, Siemens Medical Solutions). Absolute ethanol (Daihan Dehydrated Alcohol Inj; DAIHAN PHARM. CO., LTD, Seoul, Korea) was injected at the lower edge first, then the needle was slightly withdrawn, and ethanol was reinjected at the midpoint and at the upper edge. The total amount of ethanol was determined based on the following formula: V (mL) = 4/3 π (γ + 0.5)3 where γ represents the radius of the tumor in centimeters (14).
When the index tumor was oriented wider than tall against the expected needle path during the ablation procedure, rather than using a sphere-shaped needle, we used two PEI needles in a single session and inserted them in a parallel orientation within the tumor. When RFA was also needed due to the aforementioned reasons, all RFA procedures were performed immediately after the PEI procedure, with an internally cooled electrode with an adjustable active tip (Proteus RF Electrode; STARmed, Ilsan, Korea) with a 200-W generator (SSP-2000; STARmed, Ilsan, Korea) under ultrasound guidance. The algorithm used for energy deposition followed the manufacturer's instructions. Multiple overlapping ablations were applied where needed to achieve an adequate ablative margin of at least 0.5 cm. Artificial ascites were introduced before the ablation procedure whenever it was needed to improve the sonic window or to decrease the level of thermal injury to the adjacent diaphragm or colon (15). At the end of the RFA procedure, the electrode path was cauterized to prevent bleeding or tumor seeding during retraction of the electrode. Generally, the PEI treatment session was repeated with 2 day intervals for up to 3 sessions per tumor. However, when the echogenic zone induced by PEI and/or RFA was large enough to cover the entire tumor and surrounding liver after one or two treatment sessions, the ablation procedure was finished.
For the evaluation of therapeutic response, all patients were transferred to CT rooms immediately after the last ablation procedure. The radiologist who performed the ablation procedure also evaluated the CT images to determine the therapeutic response and occurrence of any immediate complications. Then, the patients were followed up with a contrast-enhanced CT one month later and then every three months after treatment.
The assessment of the safety and therapeutic efficacy was based on a proposed standardization of terminology and reporting criteria of image-guided tumor ablation (16). For the assessment of safety, we defined major complications as events that led to substantial morbidity and disability, increased level of care, or substantial lengthening of hospital stay (16). All other complications were deemed to be minor. In terms of bile duct injury, any disproportional dilatation of intrahepatic bile ducts, in the upstream of the ablated area, that was newly detected on the follow-up CT or MRI after PEI, was defined as a bile duct dilatation (17).
For evaluation of therapeutic efficacy, we evaluated the technical success rate and local tumor progression rate. Technical success was defined as an index tumor that was treated according to the protocol and covered completely based on the immediate CT scan, as determined at the time of the procedure. Local tumor progression was defined as the appearance over the follow-up of foci of an untreated disease in tumors that were previously considered to be completely ablated (16).
The patients were divided into two groups: PEI alone group versus combined PEI and RFA group. The two patient groups were compared with respect to age, sex, Child-Pugh classification, the size and location of the tumor, the number of treatment sessions, total amount of absolute ethanol used, complication rate, and therapeutic response. The presence of significant differences was determined with the Mann-Whitney test for continuous variables and with the Fisher's exact test for categorical variables. A value of p < 0.05 was considered to indicate a statistically significant difference.
There were 12 patients in the PEI alone group and 8 in the combined PEI and RFA group. The baseline patient and tumor characteristics of the two groups are summarized in Table 1. Although the mean size of the tumor in the PEI alone group was smaller than that in the combined PEI and RFA group (1.5 ± 0.3 cm vs. 2.1 ± 1.3 cm), the difference did not reach statistical significance (p = 0.11). The tumor location was mostly perivascular and was not different between the two groups.
Therapeutic outcome of the PEI alone group and the combined PEI and RFA group are also summarized in Table 1. The number of treatment sessions and the total amount of absolute ethanol used in the PEI group were greater than those of the combined PEI and RFA group (1.8 ± 0.6 vs. 1.1 ± 0.4, p = 0.04; 10.3 ± 4.8 mL vs. 5.8 ± 2.5 mL, p = 0.03), respectively. Technical success was achieved in all patients in both groups. During the follow-up period (PEI group: 23.6 ± 10.8 months; combined PEI and RFA group: 24.5 ± 9.4 months), local tumor progression was observed in 12.5% (1/8) of the combined PEI and RFA group, whereas it was found in 41.7% (5/12) of the PEI alone group (p = 0.32).
In terms of complications, bile duct dilatation was most common in both groups, as it was mostly detected on the CT obtained 1 month after the treatments. When the index tumor was in a periportal location, bile duct dilatation was common in both the PEI alone group (55.6%, [5/9]) and the combined PEI and RFA group (50.0%, [2/4]) (p = 1.00). Although bile duct dilatation was a minor complication in most patients, one patient receiving PEI alone treatment suffered from severe biliary stricture and upstream bile duct dilatation as a major complication (Fig. 1). Eventually, the bile duct dilatation progressed and atrophy of the left hepatic lobe developed in this patient. In one patient who underwent combined PEI and RFA, an abscess and cholangitis developed as a major complication (Fig. 2). In this patient, antibiotic therapy and abscess drainage was necessary. In terms of tumors contacting the gallbladder, duodenum or colon, major complications such as perforation was not found in both groups.
In this study, we have conducted PEI alone or in combination with RFA for HCCs in high risk locations. The group that received combined PEI and RFA had a lower rate of local tumor progression compared with the PEI alone group (12.5%, 1/8 vs. 41.7%, 5/12, p = 0.32), although there was no statistically significant difference, possibly due to the small sample size. Since all the studies comparing RFA to PEI were in favor of RFA, in terms of shortness of treatment sessions, local efficacy, and survival (5, 18-20), good results of combined PEI and RFA compared to PEI alone seem to be reasonable. Nevertheless, considering the small size (1.5 ± 0.3 cm) of the HCCs in the PEI alone group in which all tumors were smaller than 2 cm in diameter, the rate of local tumor progression of the PEI alone group seems to be less satisfactory than expected. Although a previous randomized controlled trial published in 2005 by Lin et al. (20) insisted that the rate of local tumor progression after PEI was comparable to that of RFA for HCCs smaller than 2 cm, it was of borderline statistical significance, and even then the PEI treated group had a higher rate of local tumor progression (PEI treated group: 48.6%, 18/37 vs. RFA treated group: 23.1%, 9/36, p = 0.06). Moreover, it has to be pointed out that RFA technology has evolved significantly since the publication of the Lin et al. (20) study and according to a previous study in which RFA was performed for a single HCC smaller than 2 cm (21), the rate of local tumor progression after RFA was found to be as low as 0.9% (2/214). Therefore, the PEI alone treatment compared to the RFA alone or combined PEI and RFA may not be as effective in terms of local tumor control, even when the size of HCCs is smaller than 2 cm. Although PEI using a multipronged injection needle with a high-dose strategy has shown promising results (14), it may not be suitable for HCC in high risk locations due to the configuration of the multipronged needle.
For HCCs in high risk locations, it has been suggested that PEI can be a good alternative treatment to RFA, due to the lower rate of complication (22-26) and this was the reason why we performed this study. However, in this study, bile duct dilatation was found as the most frequent complication after treatment for HCCs in the periportal location in both groups (PEI alone group: 55.6%, 5/9; combined PEI and RFA group: 50.0%, 2/4). The occurrence of bile duct dilatation in this study is much higher than that (3.8%, 2/52) of combined PEI and RFA by Wong et al. (11) and that (7.6%, 6/79) of RFA by Teratani et al. (4). This can be explained by the difference in the inclusion criteria of the three studies, which means the definition of the high risk location was different between the three studies (this study: tumors directly in contact with large vessels or extrahepatic vital organ; the study by Wong et al. (11): tumors within 10 mm of large vessels or extrahepatic vital organ; and the study by Teratani et al. (4): tumors within 5 mm of large vessels or extrahepatic vital organ). Even though bile duct dilatation occurs, if the tumor is located near a peripheral portal vein, the damage is minimal (27). What is of concern is the risk of central bile duct injury when the tumor is located in the hilar liver. According to a recent study (17), bile duct dilatations affecting two or more hepatic sub-segments can be regarded as a major complication of RFA, which is associated with patients' survival. In this study, biliary stricture and upstream bile duct dilatation in one patient eventually resulted in atrophy of the left hepatic lobe after PEI of hilar HCC (Fig. 1).
Severe biliary complications after PEI are not new, and in fact, an old article published in the early era of PEI warned about the possibility of such severe complications after PEI (28). The exact pathogenesis of bile duct dilatation after PEI is not known exactly, but previous authors mentioned that, after ethanol injection, gradual inflammation involving the tumor's surrounding tissues, including the bile duct, may be the cause. Another possibility of biliary stricture after PEI is the unexpected spread of ethanol into the bile duct which might result in bile duct injury. This is due to the nature of PEI itself: inhomogeneous and less predictable spread of ethanol a drawback of this procedure.
Unfortunately, however, after PEI has been replaced by RFA as the primary locoregional treatment for hepatic tumors, these severe complications seem to have been forgotten and research for these severe complications was neglected. Recently, since it is thought to be generally safe, PEI as an alternative treatment of RFA for HCCs in high risk locations has drawn attention for developing lesser complications. However, the present study demonstrates that PEI may not always be as safe as we generally believe for HCC in the hilar location, which could be contacting the central bile duct. In addition, hilar HCC as a high risk location for RFA may be high risk for PEI, as well.
The relationship between the index tumor and the intrahepatic bile duct is sometimes poorly discernable, although the location of the intrahepatic bile duct is surrogated by the neighboring portal vein. If the index tumor is contacting a large portal vein, we believe that the hepatobiliary phase of MRI obtained at 20 min after administration of gadoxetic acid would be greatly helpful for identification of the intrahepatic bile duct, since the bile duct is usually shown as a branching linear structure with high signal intensity (Fig. 1). Since PEI is also risky for HCC located in the hilar liver around the central bile duct, RFA with cooling by perfusion of ice-cold water through the endoscopic nasobiliary drainage tube would be one of the alternative treatment strategies (29, 30).
There are several limitations in this study. First, this was a retrospective study of a single institution. Therefore, the treatment protocol including the number of PEI needles and PEI sessions and the additional use of RFA was not standardized, which might have caused bias. Nevertheless, PEI to HCC in the periportal location is likely to be as risky as RFA, and may result in a bile duct complication. Second, there were not enough patients included in our study. This is mainly due to the fact that PEI is not commonly used for treatment of HCC in our institute, after experiencing severe complications and a high rate of local tumor progression. The different rate of local tumor progression between the PEI alone group and the combined PEI and RFA group may not have been statistically significant due to the small sample size. If there were a larger number of patients included, these results might have shown statistical significance. Third, the mean follow-up period was approximately 24 months for each group, which may be short for the evaluation of local tumor progression.
In conclusion, although the difference was not statistically significant, combined PEI and RFA treatment has a tendency to be more effective than PEI alone for the treatment of HCCs in high risk locations. Furthermore, even though PEI is generally accepted as a safe procedure, it may cause major biliary complications for HCCs contacting the portal vein.
Figures and Tables
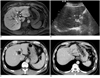 | Fig. 11.8 cm sized hepatocellular carcinoma (HCC) in 47-year-old man with liver cirrhosis due to chronic hepatitis B viral infection. He had no previous treatment history for HCC.
A. Axial hepatobiliary phase MRI image (repetition time/echo time, 4.4/2.1 ms) obtained 20 minutes after administration of gadoxetic acid shows 1.8 cm sized HCC lesion in hepatic segment 4, as low signal intensity (arrowheads). Tumor is in contact with hilar bile ducts shown as high signal intensity (arrows). B. On ultrasonogram, tumor in hepatic segment 4 is seen as low echogenecity (arrowheads), in contact with left portal vein at hilar level. Although bile duct is not delineated on this image, it is highly likely that this tumor is contacting left hilar bile duct, as well. C. Axial portal phase CT image obtained immediately after percutaneous ethanol injection (PEI) shows complete ablation of HCC (arrowheads) with no residual tumor. D. Although not shown here, liver CT taken 1 month after PEI demonstrated dilatation of left intrahepatic bile duct. Axial portal phase image of dynamic liver CT obtained 10 months after PEI shows progression of bile duct dilatation, which resulted in atrophy of left hepatic lobe.
|
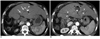 | Fig. 261-year-old man with underlying liver cirrhosis due to chronic hepatitis B viral infection who had previous treatment history of right hemihepatectomy followed by multiple episodes of transcatheter arterial chemoembilization and radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC).
A. Axial arterial phase image of dynamic liver CT shows accumulated iodized oil (black arrowhead) in left lateral segment. Anterior to accumulated iodized oil there is ill-defined enhancing area with maximum diameter of 1.5 cm, suggesting viable HCC (white arrowheads). This enhancing lesion is contacting left portal vein (arrows). Combined percutaneous ethanol injection (PEI) and RFA was conducted with technical success. B. Patient complained of abdominal pain and fever about 40 days after combined PEI and RFA. Axial arterial phase image of dynamic liver CT taken for evaluation demonstrates no evidence of local tumor progression, but abscess demonstrates formation in ablated zone (black arrowheads). Dilatation of intrahepatic bile ducts with wall enhancement is also seen (white arrowheads), suggesting cholangitis.
|
Table 1
Comparison between PEI Alone Group and Combined PEI and RFA Group

Note.- *Mann-Whitney test was used, †Fisher's exact test was used, ‡One tumor was abutting to duodenum, colon, and IVC, §In one patient, both bile duct dilatation and abscess were developed after combined PEI and RFA. PEI = percutaneous ethanol injection, RFA = radiofrequency ablation, SD = standard deviation, PV = portal vein, HV = hepatic vein, IVC = inferior vena cava, GB = gallbladder
References
1. Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet. 1998. 351:214–215.
2. El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999. 340:745–750.
3. Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997. 350:1142–1143.
4. Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006. 43:1101–1108.
5. Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005. 129:122–130.
6. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004. 127:1714–1723.
7. Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991. 68:1524–1530.
8. Galandi D, Antes G. Radiofrequency thermal ablation versus other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2002. CD003046.
9. Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005. 234:954–960.
10. Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003. 10:67–76.
11. Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. AJR Am J Roentgenol. 2008. 190:W187–W195.
12. Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Funaki T, Arima K, et al. Combination therapy of percutaneous ethanol injection and radiofrequency ablation against hepatocellular carcinomas difficult to treat. Int J Oncol. 2002. 21:611–615.
13. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011. 53:1020–1022.
14. Kuang M, Lu MD, Xie XY, Xu HX, Xu ZF, Liu GJ, et al. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 2009. 253:552–561.
15. Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites. Abdom Imaging. 2009. 34:371–380.
16. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009. 20:7 Suppl. S377–S390.
17. Kondo Y, Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, et al. Intrahepatic bile duct dilatation after percutaneous radiofrequency ablation for hepatocellular carcinoma: impact on patient's prognosis. Liver Int. 2011. 31:197–205.
18. Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009. 104:514–524.
19. Bouza C, López-Cuadrado T, Alcázar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009. 9:31.
20. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005. 54:1151–1156.
21. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008. 47:82–89.
22. Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: Current status. World J Radiol. 2010. 2:417–424.
23. Livraghi T, Meloni F, Morabito A, Vettori C. Multimodal image-guided tailored therapy of early and intermediate hepatocellular carcinoma: long-term survival in the experience of a single radiologic referral center. Liver Transpl. 2004. 10:2 Suppl 1. S98–S106.
24. Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003. 226:441–451.
25. Kwon JH. Is percutaneous ethanol injection therapy still effective for hepatocellular carcinoma in the era of radiofrequency ablation? Gut Liver. 2010. 4:Suppl 1. S105–S112.
26. Sung YM, Choi D, Lim HK, Lee WJ, Kim SH, Kim MJ, et al. Long-term results of percutaneous ethanol injection for the treatment of hepatocellular carcinoma in Korea. Korean J Radiol. 2006. 7:187–192.
27. Kim SH, Lim HK, Choi D, Lee WJ, Kim SH, Kim MJ, et al. Changes in bile ducts after radiofrequency ablation of hepatocellular carcinoma: frequency and clinical significance. AJR Am J Roentgenol. 2004. 183:1611–1617.
28. Koda M, Okamoto K, Miyoshi Y, Kawasaki H. Hepatic vascular and bile duct injury after ethanol injection therapy for hepatocellular carcinoma. Gastrointest Radiol. 1992. 17:167–169.
29. Ogawa T, Kawamoto H, Kobayashi Y, Nakamura S, Miyatake H, Harada R, et al. Prevention of biliary complication in radiofrequency ablation for hepatocellular carcinoma-Cooling effect by endoscopic nasobiliary drainage tube. Eur J Radiol. 2010. 73:385–390.
30. Lam VW, Ng KK, Chok KS, Cheung TT, Wat J, Fan ST, et al. Safety and efficacy of radiofrequency ablation for periductal hepatocellular carcinoma with intraductal cooling of the central bile duct. J Am Coll Surg. 2008. 207:e1–e5.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download