Abstract
Objective
To report the feasibility of magnetic resonance imaging (MRI)-guided intervention for diagnosing suspicious breast lesions detectable by MRI only, using the freehand technique with a 3.0-T closed-bore MRI scanner.
Materials and Methods
Five women with 5 consecutive MRI-only breast lesions underwent MRI-guided intervention: 3 underwent MRI-guided needle localization and 2, MRI-guided vacuum-assisted biopsy. The interventions were performed in a 3.0-T closed-bore MRI system using a dedicated phased-array breast coil with the patients in the prone position; the freehand technique was used. Technical success and histopathologic outcome were analyzed.
Results
MRI showed that four lesions were masses (mean size, 11.5 mm; range, 7-18 mm); and 1, a nonmass-like enhancement (maximum diameter, 21 mm). The locations of the lesions with respect to the breast with index cancer were as follows: different quadrant, same breast - 3 cases; same quadrant, same breast - 1 case; and contralateral breast - 1 case. Histopathologic evaluation of the lesions treated with needle localization disclosed perilobular hemangioma, fibrocystic change, and fibroadenomatous change. The lesions treated with vacuum-assisted biopsy demonstrated a radial scar and atypical apocrine hyperplasia. Follow-up MRI after 2-7 months (mean, 4.6 months) confirmed complete lesion removal in all cases.
Magnetic resonance imaging (MRI) of the breast can be used to detect mammographically and clinically occult breast cancer. Although MRI has a high reported sensitivity approaching 100% for breast cancer detection, the reported specificity is rather low, ranging from 37% to 97% (1-4).
When suspicious, enhancing breast lesions are detected by MRI alone, MRI-guided interventions are required for tissue sampling and histopathologic diagnosis of the lesions. MRI-guided tissue sampling of these so-called "MRI-only lesions" can be performed by needle localization followed by surgical excision, MRI-guided large-core needle biopsy, or vacuum-assisted biopsy (5).
Various methods have been described in the literature for MRI-guided breast biopsy. For example, the freehand technique and the stereotactic technique use a grid or compression plate (6-9). The freehand technique has several advantages over the stereotactic technique such as its simplicity and it does not require specialized grid devices. Further, it allows for the needle to be angled freely, enabling localization of lesions throughout the breast, from near the chest wall to superficial locations near the nipple; further, it also enables localization of lesions in patients with silicone implants (6). Additionally, since a grid is not required, the breast need not be compressed between compression plates during localization, a process that sometimes changes the lesion location and interferes with the contrast enhancement of the lesion (5, 10).
Thus far, several investigators have described their clinical experiences with MRI-guided interventions performed using the freehand technique (6-8, 11-15). However, these reports pertain almost exclusively to Western countries, and to the best of our knowledge, no reports have been published on experiences in Korea. Moreover, most previous studies have focused on the utility of an open MRI system and have used a 1.0- or 1.5-tesla (T) MRI system.
The purpose of this feasibility study is to report our experience with MRI-guided needle localization and vacuum-assisted biopsy using the freehand technique in a 3.0-T closed-bore MRI scanner for the diagnosis of breast lesions visible only on MRI.
From August 2009 to October 2010, our MRI database was retrospectively reviewed to identify consecutive patients with suspicious lesions initially detected with breast MRI who had undergone MRI-guided intervention. Written informed consent was obtained from all the study subjects, and this retrospective study was approved by the institutional review board of our institution.
All breast MRI scans were interpreted using the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) MRI lexicon by a single breast radiologist with 8.4 years of experience (16, 17). For lesions that were interpreted as suspicious (BI-RADS category 4) or highly suggestive of malignancy (BI-RADS category 5) on the basis of their MRI morphologic and kinetic features, a targeted second-look ultrasound (US) was performed by the interpreting radiologist. If the lesion was reliably identified by US or mammography, a biopsy was performed under the guidance of US or mammography. If the lesion was not seen with US, MRI-guided intervention was recommended. The biopsy results were correlated with the MRI findings. In case of discordance, an additional biopsy was recommended. This was also recommended if the biopsy result showed atypia or a high-risk lesion such as a radial scar or papilloma.
Diagnostic MRI was performed using a 3-T closed-bore MRI system (Achieva, Philips Medical Systems, Best, The Netherlands) with a dedicated phased-array bilateral breast coil (MRI devices, Wurzburg, Germany). The gadolinium contrast agent Omniscan (gadodiamide; GE Healthcare Milwaukee, WI, USA) was used: 20 mL was hand injected followed by a 10 mL saline flush. The protocol included axial noncontrast T1-weighted fast spin echo imaging (485.22/9.48, repetition time [TR] [ms]/echo time [TE] [ms]), bilateral sagittal fast spin echo T2-weighted fat-suppressed imaging (4081/70, TR [ms]/TE [ms]), and dynamic contrast-enhanced fat-suppressed T1-weighted imaging. The parameters for the latter were TR/TE, 4.2/1.7 (ms); flip angle, 10°; FOV, 200 × 200 mm; and dynamic scan duration, 60 s. The section thickness was 2.0 mm with a 344 × 345 matrix. Precontrast and 5 consecutive postcontrast images were captured at an interval of 60 s. Subtraction images were obtained by subtracting the precontrast images from each serial postcontrast image on a pixel-by-pixel basis.
Needle localization was performed with the patient in the prone position by using a dedicated surface breast coil. In all cases, the procedure was performed via the freehand technique, as first described by Daniel et al. (6), by a single radiologist who was a specialist in breast imaging. The procedure was initiated by fixing a skin marker (fiducial marker doped with gadolinium or a vitamin E capsule) to the skin of the breast overlying the approximate position of the lesion. The coordinates of the skin marker relative to the target were judged from an axial noncontrast T1-weighted spin-echo image. The target lesion was identified on the noncontrast T1-weighted image by using breast architecture as a map. To determine the optimal needle entry site on the skin, the skin marker was repositioned and the images were rescanned until both the center of the skin marker and the center of the suspected target were visualized on the same axial image. When the skin marker was correctly positioned, the patient was removed from the system. After detaching the skin marker from the skin, the needle entry point on the skin was marked with a pen and sterilized with povidone-iodine. The skin was draped, and 10 mL of a superficial, sub-dermal anesthetic (1% lidocaine) was injected. A titanium MRI-compatible 18G needle (Somatex Medical Technologies GmbH, Teltow, Germany) was inserted into the entry site marked on the skin and directed along the planned needle approach. The MRI table was returned to the bore of the magnet, and an axial image was obtained. The needle was repositioned until the image showed its tip to be at the edge of the target (Figs. 1, 2). In order to confirm the accurate placement of the needle, postcontrast axial and sagittal fat-suppressed T1-weighted images were obtained as dynamic sequences of the diagnostic breast MRI. An intravenous bolus of 20-mL gadolinium contrast agent Omniscan (gadodiamide; GE Healthcare Milwaukee, WI, USA) was administered by hand injection followed by a 10-mL saline flush. After placement was confirmed, the MRI table was removed from the system. The outer sheath of the needle was removed, and an MRI-compatible hookwire was deployed. Axial and sagittal fat-suppressed T1-weighted images were captured to confirm the accurate placement of the wire. The patient was then transferred to the operation room, and an excisional biopsy was performed at the localized site (Fig. 3).
For this procedure, the lesion localization method and MRI technique were the same as those in the MRI-guided needle localization procedure. After correct placement of the coaxial needle was confirmed, an MR-compatible vacuum-assisted breast biopsy needle (Vacora vacuum-assisted biopsy system, Bard Biopsy Systems, Karlsruhe, Germany; or Mammotome Hand Held, Ethicon Endo-Surgery, Cincinnati, OH, USA) was inserted through the coaxial sheath, and the biopsy was performed (Fig. 1). Over 10 samples were obtained, and axial and sagittal fat-suppressed T1-weighted images were performed to confirm the success of the procedure (Fig. 2).
Between August 2009 and October 2010, 438 patients underwent bilateral breast MRI for the evaluation of preoperative staging in recently diagnosed breast cancer. Among these, 91 patients had 93 additional suspicious lesions as detected by MRI. A second-look US was conducted for all lesions. Finally, 5 lesions in 5 patients were not detected in the second-look US, and MRI-guided intervention was conducted for these 5 MRI-only suspicious lesions (Table 1). MRI-guided needle localization was conducted for 3 of the 5 lesions and MRI-guided vacuum-assisted biopsy, for 2.
The average patient age was 52 years (range, 40-60 years). The types of MRI-enhancing lesions were masses in 4 of the 5 cases (80%) and a nonmass-like enhancement in 1 case (20%). The mean size of the masses was 11.5 mm (range, 7-18 mm), and the maximum diameter of the nonmass-like enhancement was 21 mm. The location of the lesions was a different quadrant of the same breast with index cancer in 3 cases (60%), the same quadrant of the same breast with index cancer in 1 (20%), and the contralateral breast in 1 (20%).
All procedures were performed successfully. The average procedure time of MRI-guided needle localization was 43 min (range, 40-50 min). The time required for MRI-guided vacuum-assisted biopsy was 20 min longer than that required for MRI-guided needle localization (average, 65 min; range, 50-80 min). The average number of times the patient was moved in and out of the magnet was 1.7 (range, 1-2) during MRI-guided needle localization and 2.5 (range, 2-3) during MRI-guided vacuum-assisted biopsy.
The pathologic results of 3 cases of MRI-guided localization followed by excision were perilobular hemangioma, fibrocystic change, and fibroadenomatous change (Table 2, Fig. 3). Those of 2 cases for MRI-guided vacuum-assisted biopsy were radial scar and atypical apocrine hyperplasia (Fig. 2). Since high-risk lesions had been identified, these 2 cases were referred to excisional biopsy following US-guided needle localization for hematomas formed at the sites of the previous biopsy. The final excisional biopsy showed no residual lesion of the radial scar or residual atypical apocrine hyperplasia. A follow-up MRI scan obtained after 2-7 months (mean, 4.6 months) showed no evidence of residual lesions or post-biopsy changes.
In this report, we present the results of an MRI-guided intervention conducted using the freehand technique with a 3.0-T closed-bore MRI system for the diagnosis of MRI-only suspicious breast lesions. MRI-guided needle localization and vacuum-assisted biopsy were successfully performed for all 5 patients. The results of this feasibility study, which demonstrated 100% accuracy, are in accordance with those of previous studies on MRI-guided biopsy, which reported technical success rates ranging from 96% to 100% (4, 5, 11-15, 18-21). Although our study included a small series, the findings suggest that this technique is feasible and accurate.
Magnetic resonance imaging-guided interventions can be performed using the freehand technique (6-8, 11-15, 22) or guidance methods, such as compression grid systems, that allow the capture of coordinates (9, 23-25). The stereotactic method using a compression grid system proved to have several limitations (23, 24). First, the grid limits access to areas located between the holes. Second, there are difficulties in localizing lesions in the retroareolar region, near the chest wall, and in the axillary tail. In contrast, the freehand technique has the advantage of allowing the needle to be angled freely, enabling localization of difficult inaccessible breast lesions as well as localization of lesions in patients with silicone implants (6). Moreover, when a grid is not used, the breast is not compressed between the compression plates. Therefore, the target lesion will not show variation between the diagnostic MRI and the images obtained during localization; furthermore, contrast enhancement of the lesion will not be reduced due to breast compression (5, 10).
However, compared to the stereotactic method using a compression grid system, the freehand technique has the potential disadvantage of a longer examination time, because repeated imaging is necessary to confirm needle placement. Open systems that allow real-time imaging may be most amenable to the freehand approach, as needle repositioning and confirming needle location can be performed faster. However, closed-bore MRI systems are more common, have higher field strength and better field homogeneity, and exhibit the maximum validation data for MRI-guided intervention. In our study, direct monitoring of the procedure was not possible because the procedure was conducted in a closed-bore MRI system, but all the lesions were localized accurately. In all cases, contrast-enhanced MRI was used to confirm the correct positioning of the needle in the target lesion. Due to the impossibility of directly monitoring needle movement, careful imaging-pathologic correlation is more important in MRI-guided core biopsy than US-guided core biopsy (26).
Magnetic resonance imaging-guided interventions can be performed with the patient in the prone or supine positions. Although the latter has been used by some investigators (22, 27), the former is usually preferred. The disadvantages of the supine position are that lesion identification is problematic because the breast configuration is different from that in the prone position adopted in the preprocedural diagnostic MRI, motion artifacts may occur, and the resolution is limited because a dedicated breast coil is not used. For these reasons, we used the prone position. Some reported that limitations of the prone position are breast motion and limited needle access because of the dedicated breast coil (28). In our study, breast motion did not occur, because the weight of the patient's prone body fixed the chest wall to the coil platform, as described previously by Meeuwis et al. (13). Further, we did not face any problems while localizing the target lesion with the breast in a dedicated breast coil, because the needle was placed tangentially.
Thus far, most previous studies on MRI-guided breast biopsy have been performed using 1.0- or 1.5-T MRI. As mentioned by Meeuwis et al. (13), imaging at 3.0-T with a dedicated breast coil facilitates accurate localization of the breast lesion on non-contrast images because of the high spatial resolution. During the procedure in the present study with a 3.0-T MRI system, all the lesions could be identified on non-enhanced T1 weighted images by using the breast architecture as a map. After the procedure, the needle position was checked using contrast-enhanced images.
If contrast-enhanced MRI is not conducted after MRI-guided biopsy, it is difficult to confirm that the correct area has been excised. An MRI-compatible clip that can remain in situ after needle localization can be placed to enable lesion to be identified. A post-operative MRI can be used to confirm successful lesion excision. Here, we did not use the clip because it is not available or commonly used in Korea; however, we did perform a follow-up MRI, which confirmed lesion removal in all 5 patients (100% accuracy).
Some previous studies on MRI-guided needle localization reported a failure rate of 3-4% (9, 12), attributing it to guidewire migration. Irrespective of whether MRI-guided localization yields benign findings or shows a high-risk lesion, short-term follow-up MRI 6 months or earlier after surgery is recommended to ensure lesion removal (5, 9, 12, 29).
This study has some limitations: It is a retrospective review of selective patients who underwent MRI-guided intervention. The number of patients examined was small as only 5 women were examined. This is because most patients who underwent MRI were recently diagnosed with breast cancer and also underwent whole-breast US before the MRI and second-look US after MRI; therefore, MRI-only lesions are rare at our institution.
In conclusion, our study showed that MRI-guided wire localization and vacuum-assisted biopsy using the freehand technique with a 3.0-T closed-bore MRI system are technically feasible and safe. In all patients, the procedure allowed accurate tissue sampling (100%). In the future, studies with a larger number of patients will be needed to validate our results before the freehand technique can be accepted in routine clinical practice for MRI-guided biopsy.
Figures and Tables
 | Fig. 1Breast MRI equipment and technique.
Patient was placed in prone position (A) on dedicated phased-array bilateral breast coil. Handheld MRI-guided biopsy device (arrows in B) was introduced into coaxial sheath (arrow in C) to acquire tissue specimens.
|
 | Fig. 260-year-old woman diagnosed with invasive ductal carcinoma in left breast after US-guided core needle biopsy.
A, B. Preoperative MRI scan shows 1.0 cm ill-defined irregular early-enhancing mass (arrow) in right breast on sagittal post Gd-enhanced fat-suppressed T1-weighted image (A) and axial non-enhanced T1-weighted image, which was not seen in second-look US. To exclude bilateral breast cancer, MRI-guided vacuum-assisted biopsy was performed. C. Noncontrast axial T1-weighted image before needle placement showed lesion with low T1 signal intensity (arrow) under skin marker (arrowhead). D, E. Nonenhanced axial T1-weighted images following needle placement showed that needle tip (arrow) was advanced to edge of lesion, ideal position for accurate sampling. F, G. Axial and sagittal contrast-enhanced images confirmed correct needle placement (arrows). H. Post-biopsy, sagittal fat-suppressed T1-weighted image showed high signal intensity surrounding needle due to hematoma, air, and anesthetic (arrows). Pathologic examination of cores indicated atypical apocrine hyperplasia. This high-risk lesion was referred to surgery following US-guided needle localization for hematoma at site of previous biopsy. No residual lesion was found on pathologic examination. US = ultrasound
|
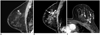 | Fig. 356-year-old woman diagnosed with invasive ductal carcinoma in left breast after US-guided core needle biopsy.
A. Sagittal T1-weighted contrast-enhanced MR image shows 11-mm ill-defined irregular early-enhancing mass (arrowhead) in different quadrant of same breast with index cancer. No corresponding lesion was detected on second-look US. To exclude multicentric growth, MRI-guided tissue sampling was performed by needle localization followed by surgical excision. B, C. Sagittal (B) and axial (C) contrast-enhanced images obtained after needle placement showed needle tip (arrow) just anterior to enhancing mass (arrowhead). Lesion was successfully localized and excisional biopsy showed fibroadenomatous changes. US = ultrasound
|
Acknowledgments
The authors thank Dr. Bruce L. Daniel for introducing and demonstrating free hand technique of MRI-guided intervention.
References
1. Orel SG, Schnall MD, LiVolsi VA, Troupin RH. Suspicious breast lesions: MR imaging with radiologic-pathologic correlation. Radiology. 1994. 190:485–493.
2. Stomper PC, Herman S, Klippenstein DL, Winston JS, Edge SB, Arredondo MA, et al. Suspect breast lesions: findings at dynamic gadolinium-enhanced MR imaging correlated with mammographic and pathologic features. Radiology. 1995. 197:387–395.
3. Boné B, Aspelin P, Bronge L, Isberg B, Perbeck L, Veress B. Sensitivity and specificity of MR mammography with histopathological correlation in 250 breasts. Acta Radiol. 1996. 37:208–213.
4. Daniel BL, Yen YF, Glover GH, Ikeda DM, Birdwell RL, Sawyer-Glover AM, et al. Breast disease: dynamic spiral MR imaging. Radiology. 1998. 209:499–509.
5. Kuhl CK, Elevelt A, Leutner CC, Gieseke J, Pakos E, Schild HH. Interventional breast MR imaging: clinical use of a stereotactic localization and biopsy device. Radiology. 1997. 204:667–675.
6. Daniel BL, Birdwell RL, Ikeda DM, Jeffrey SS, Black JW, Block WF, et al. Breast lesion localization: a freehand, interactive MR imaging-guided technique. Radiology. 1998. 207:455–463.
7. Brenner RJ, Shellock FG, Rothman BJ, Giuliano A. Technical note: magnetic resonance imaging-guided pre-operative breast localization using "freehand technique". Br J Radiol. 1995. 68:1095–1098.
8. Lee CH, Smith RC, Levine JA, Troiano RN, Tocino I. Clinical usefulness of MR imaging of the breast in the evaluation of the problematic mammogram. AJR Am J Roentgenol. 1999. 173:1323–1329.
9. Morris EA, Liberman L, Dershaw DD, Kaplan JB, LaTrenta LR, Abramson AF, et al. Preoperative MR imaging-guided needle localization of breast lesions. AJR Am J Roentgenol. 2002. 178:1211–1220.
10. Kuhl CK, Bieling H, Gieseke J, Ebel T, Mielcarek P, Far F, et al. Breast neoplasms: T2* susceptibility-contrast, first-pass perfusion MR imaging. Radiology. 1997. 202:87–89.
11. Daniel BL, Birdwell RL, Butts K, Nowels KW, Ikeda DM, Heiss SG, et al. Freehand iMRI-guided large-gauge core needle biopsy: a new minimally invasive technique for diagnosis of enhancing breast lesions. J Magn Reson Imaging. 2001. 13:896–902.
12. van den Bosch MA, Daniel BL, Pal S, Nowels KW, Birdwell RL, Jeffrey SS, et al. MRI-guided needle localization of suspicious breast lesions: results of a freehand technique. Eur Radiol. 2006. 16:1811–1817.
13. Meeuwis C, Peters NH, Mali WP, Gallardo AM, van Hillegersberg R, Schipper ME, et al. Targeting difficult accessible breast lesions: MRI-guided needle localization using a freehand technique in a 3.0 T closed bore magnet. Eur J Radiol. 2007. 62:283–288.
14. Hauth EA, Jaeger HJ, Lubnau J, Maderwald S, Otterbach F, Kimmig R, et al. MR-guided vacuum-assisted breast biopsy with a handheld biopsy system: clinical experience and results in postinterventional MR mammography after 24 h. Eur Radiol. 2008. 18:168–176.
15. van de Ven SM, Lin MC, Daniel BL, Sareen P, Lipson JA, Pal S, et al. Freehand MRI-guided preoperative needle localization of breast lesions after MRI-guided vacuum-assisted core needle biopsy without marker placement. J Magn Reson Imaging. 2010. 32:101–109.
16. American College of Radiology (ACR). BI-RADS® Breast Imaging Reporting and Data System, Breast Imaging Atlas. 2003. Reston, VA: American College of Radiology.
17. Ikeda DM, Hylton NM, Kinkel K, Hochman MG, Kuhl CK, Kaiser WA, et al. Development, standardization, and testing of a lexicon for reporting contrast-enhanced breast magnetic resonance imaging studies. J Magn Reson Imaging. 2001. 13:889–895.
18. Veltman J, Boetes C, Wobbes T, Blickman JG, Barentsz JO. Magnetic resonance-guided biopsies and localizations of the breast: initial experiences using an open breast coil and compatible intervention device. Invest Radiol. 2005. 40:379–384.
19. Fischer U, Kopka L, Grabbe E. Magnetic resonance guided localization and biopsy of suspicious breast lesions. Top Magn Reson Imaging. 1998. 9:44–59.
20. Pfleiderer SO, Reichenbach JR, Azhari T, Marx C, Wurdinger S, Kaiser WA. Dedicated double breast coil for magnetic resonance mammography imaging, biopsy, and preoperative localization. Invest Radiol. 2003. 38:1–8.
21. Smith LF, Henry-Tillman R, Rubio IT, Korourian S, Klimberg VS. Intraoperative localization after stereotactic breast biopsy without a needle. Am J Surg. 2001. 182:584–589.
22. Fischer U, Vosshenrich R, Keating D, Bruhn H, Döler W, Oestmann JW, et al. MR-guided biopsy of suspect breast lesions with a simple stereotaxic add-on-device for surface coils. Radiology. 1994. 192:272–273.
23. Heywang-Köbrunner SH, Heinig A, Pickuth D, Alberich T, Spielmann RP. Interventional MRI of the breast: lesion localisation and biopsy. Eur Radiol. 2000. 10:36–45.
24. Orel SG, Schnall MD, Newman RW, Powell CM, Torosian MH, Rosato EF. MR imaging-guided localization and biopsy of breast lesions: initial experience. Radiology. 1994. 193:97–102.
25. Carbognin G, Girardi V, Brandalise A, Baglio I, Bucci A, Bonetti F, et al. MR-guided vacuum-assisted breast biopsy in the management of incidental enhancing lesions detected by breast MR imaging. Radiol Med. 2011. 116:876–885.
26. Youk JH, Kim EK, Kim MJ, Ko KH, Kwak JY, Son EJ, et al. Concordant or discordant? Imaging-pathology correlation in a sonography-guided core needle biopsy of a breast lesion. Korean J Radiol. 2011. 12:232–240.
27. Döler W, Fischer U, Metzger I, Harder D, Grabbe E. Stereotaxic add-on device for MR-guided biopsy of breast lesions. Radiology. 1996. 200:863–864.
28. Coulthard A. Magnetic resonance imaging-guided preoperative breast localization using a "freehand technique". Br J Radiol. 1996. 69:482–483.
29. Kuhl CK, Morakkabati N, Leutner CC, Schmiedel A, Wardelmann E, Schild HH. MR imaging--guided large-core (14-gauge) needle biopsy of small lesions visible at breast MR imaging alone. Radiology. 2001. 220:31–39.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download