Abstract
We report a case of intraocular lymphoma in a 65-year-old man, 15 months after cardiac transplantation. On Magnetic Resonance (MR) images, the iris and the anterior chamber of the right eye were found to be involved with an enhancing soft-tissue lesion. To our knowledge, this is the first case of post-transplantation intraocular lymphoma evaluated with MR imaging.
Posttransplantation lymphoproliferative disorder (PTLD) is an opportunistic lymphocytic disorder that occurs in the setting of immunosuppression after organ transplantation. It encompasses a heterogeneous group of diseases, ranging from benign polyclonal polymorphic lymphocyte hyperplasia to monoclonal lymphoma (1, 2). Intraocular PTLD is rare and only a few cases have been reported in the English literature (3-6). It can masquerade as uveitis that is unresponsive to topical steroid therapy (3-5), and a high index of suspicion is crucial for the early diagnosis of patients with a history of organ transplantation. We report the On Magnetic Resonance (MR) imaging findings of isolated intraocular lymphoma in a 65-year-old man who underwent cardiac transplantation 15 months ago. To our knowledge, this is the first reported case of posttransplantation intraocular lymphoma evaluated with MR imaging.
A 64-year-old diabetic man underwent cardiac transplantation for ischemic cardiomyopathy in September 2006. He was maintained on immunosuppressive therapy with tacrolimus, mycophenolate, and prednisolone. In April 2007, he visited a local clinic with a complaint of decreased vision in the right eye that had persisted for 3 months and was diagnosed with diabetic retinopathy complicated by cataract and vitreous hemorrhage. He underwent phacoemulsification with posterior chamber lens implantation, pars plana vitrectomy, membrane peeling, endolaser, and intravitreal injection of Avastin (Bevacizumab, Genentech/Roche, San Francisco, CA, USA). Despite the operation, the visual acuity continued to rapidly decline and with a diagnosis of endophthalmitis, the patient was referred to our ophthalmologic department in June 2007. On initial examination, the patient was afebrile and denied weight loss, gastrointestinal trouble, headache, or sore throat. The visual acuity was 20/400 oculus dexter and 20/30 oculus sinister. Slit lamp examination showed 4+ cells in the right anterior chamber. A thick, turbid material was drawn by vitreous tapping, but not specific microorganisms were isolated on culturing this material. Intravitreal injection and topical application of antibiotics did not improve the patient's clinical symptoms. In August 2007, the patient underwent vitrectomy, endolaser, and removal of the intraocular lens and he received intravitreal injection of antibiotics but showed no clinical improvement. In November 2007, the visual acuity of the right eye declined to hand movement, and ophthalmologic examination revealed an intraocular mass at the iridocorneal angle and anterior chamber of the right eye. Cytologic preparations of the anterior chamber aspirate revealed a few atypical lymphoid cells and many apoptotic cells, suggestive of PTLD.
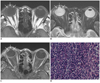
Magnetic resonance (MR) imaging was performed to further characterize the lesion and demonstrated a smooth, undulating soft-tissue lesion in the iris and the anterior chamber in the right eye. Compared with the vitreous humor, the lesion was hyperintense on T1- (Fig. 1A) and hypointense on T2-weighted images (Fig. 1B), and it was well enhanced after the administration of contrast material (Fig. 1C). The transplantation team and the ophthalmologist made a joint decision to perform enucleation, because the ocular infection was intractable to conventional therapy and the recovery of visual acuity was deemed unlikely. Routine histopathological analysis and immunohistochemical staining of the ocular mass showed an extensive mononuclear cell infiltrate with large cells and a vesicular chromatic pattern, consistent with diffuse large B-cell lymphoma (Fig. 1D). In situ tissue hybridization demonstrated a substantial fraction of latent Epstein-Barr virus (EBV). In October 2010, the patient died of heart and renal failure complicated by sepsis from pneumonia and cholecystitis.
Posttransplantation lymphoproliferative disorder (PTLD) is a serious complication of solid organ transplantation and represents a spectrum of unregulated lymphoid proliferation that ranges from polyclonal hyperplasia to monoclonal malignant lymphoma (7). The classification scheme of PTLD is constantly evolving and currently includes 4 major categories: hyperplastic (or early) lesions; polymorphic (generally monoclonal) lesions; monomorphic (lymphomatous, invariably monoclonal) lesions, which are further subcategorized along recognized lines of B-cell, T-cell, or natural killer cell neoplasia; and other lymphoproliferative disorders, including Hodgkin lymphoma (2). Approximately 85% of cases are of B-cell origin and are related to EBV, as was the case with our patient (7). The pathogenesis of EBV-related PTLD is considered to be the result of the failure of the host immune system to defend against EBV infection by limiting the T-cell responses that normally control the proliferation of infected B-cells (4). In immunocompetent individuals, massive B-cell proliferation induced by EBV infection eventually subsides under the control of an equally massive cytotoxic T-cell reaction (2). However, under conditions of immunosuppression, defective T-cell function may allow uncontrolled proliferation of EBV-infected B-cells, which express a set of viral genes known as "latency" genes. These genes make EBV-infected B-cells autonomously proliferate and form hyperplastic, polyclonal lesions. When polyclonal proliferation continues, the lesions progress to monoclonality, eventually forming a malignant lymphoma (1, 2). However, this hypothesis does not explain the existence of EBV-negative and T-cell or natural killer cell-derived PTLDs.
The frequency of PTLD has been reported to range from 1% to 10%, and it differs with the type of organ transplanted and the type and length of immunosuppression. The highest prevalence has been recorded in multivisceral transplant recipients (13-33% cases), followed by bowel (7-11%), heart-lung (9.4%), lung (1.8-7.9%), heart (3.4%), liver (2.2%), and kidney (1%) recipients (2, 8, 9). The risk of PTLD is greater with primary EBV infection posttransplantation, an organ from an EBV-seropositive donor transplanted into an EBV-seronegative recipient, increased circulating levels of EBV genome, and the presence of cytomegalovirus infection (1, 2). The prevalence of PTLD is also greater in the pediatric population and in patients over 60 years of age. The site of occurrence of PTLD is also influenced by the organ allografts (2), and lesions most commonly occur in the gastrointestinal tract, central nervous system, and allografted organs, and, less commonly, in the lymph nodes (8). Involvement of the extracranial head and neck in PTLD is reportedly uncommon and is usually seen in association with disseminated extranodal disease (2, 10). Most commonly, the disease presents as a focal mass in the Waldeyer ring and cervical lymphadenopathy. Less commonly, the orbit and sinonasal tract can be involved (2, 8, 10). Sinonasal disease mimics fungal sinusitis or nasal polyposis, manifesting as a bulky mass with bone erosion and invasion of the orbits. Orbital involvement may manifest as a soft-tissue mass with a propensity for the lacrimal gland fossa, often accompanied by bone destruction (2, 10).
Intraocular PTLD is rare, and to the best of our knowledge, less than 20 cases have been reported in the English literature (3-6). Patients present with various clinical symptoms including decreased vision, a mass in the iris, uveitis/papillitis, eye discoloration, photosensitivity, diplopia, ophthalmoplegia, and corneal opacity. In a metaanalysis of 13 reported cases of intraocular PTLD, Fujita et al. (6) found that most cases (12/13, 92%) involved pediatric patients and the lesion frequently involved the anterior chamber and iris. They also noted that liver transplant patients were more commonly affected (8/13, 62%). Although older than patients in previous studies, our patient also had a lesion in the iris and anterior chamber.
The most important differential diagnoses of adult ocular masses include ocular melanoma and metastasis. Ocular melanoma is the most common primary malignant intraocular tumor in adults, accounting for 80% of noncutaneous melanomas (11). Like other melanin-containing tumors, ocular melanoma shows a high signal intensity on T1-weighted images and low signal intensity on T2-weighted images. However, since ocular lymphoma may have signal characteristics similar to those of uveal melanomas, MR signal characteristics may not be helpful for the differential diagnosis (11). Instead, morphologic features may aid differential diagnosis, since iris melanoma tends to present as a discrete circumscribed lesion rather than a plaque-like thickening, as seen in iris lymphomas such as the present case (12). Metastatic eye disease occurs most commonly from the lung and breast. Although metastases may produce solitary lesions, bilaterality and multicentricity usually suggest metastases. Other rare primary tumors to be differentiated from ocular lymphoma include medulloepithelioma, uveal leiomyoma, retinal pigment epithelial adenoma and adenocarcinoma, and optic disk melanocytoma. Medulloepithelioma and uveal leiomyomas tend to occur in the ciliochoroidal region and may be difficult to differentiate from the lymphoma seen in the present case on the basis of the imaging findings alone. Medulloepithelioma is a congenital tumor of the uvea, which presents as a mass behind the iris and pupil. Uveal leiomyoma is even rarer than other intraocular tumors and affects the ciliary body and the choroid overlying the posterior pole (12).
As seen in the present case, the clinical diagnosis of ocular PTLD is often difficult, because it can masquerade as steroid-resistant uveitis (3, 4). In such situations, MR imaging may provide a diagnostic clue by revealing abnormal thickening of the uvea, especially in the anterior segment, with pathologic contrast enhancement. Although the imaging findings of uveitis are nonspecific, an inflamed uveal tract usually shows a high signal intensity on T2-weighted images in contrast to most ocular tumors, which present a low to intermediate signal intensity, as in the present case (13). Although our patient died of heart and renal failure complicated by sepsis 11 months after the diagnosis of posttransplantation intraocular lymphoma, the clinical course of ocular PTLD is chronic and the reported prognosis is generally good (6). Treatment includes reduction of immunosuppression, antiviral agents, and radiation therapy.
In summary, intraocular lymphoma can occur as a rare manifestation of PTLD. A high index of suspicion should be used for early diagnosis and treatment, when abnormal thickening of the uvea with contrast enhancement is seen on MR images in patients with a history of organ transplantation.
Figures and Tables
Fig. 1
Posttransplantation intraocular lymphoma.
A-C. MR images showing smooth, undulating soft-tissue lesion in iris and anterior chamber in right eye (arrows). Compared with vitreous humor, lesion is hyperintense on T1-weighted image (A), hypointense on T2-weighted image (B), and well enhanced after administration of contrast material (C). D.. Photomicrograph showing dense mononuclear large cell infiltrate, vesicular chromatic pattern, and prominent nucleoli, consistent with diffuse large B-cell lymphoma (hematoxylin-eosin; original magnification, × 20).
References
1. Dolcetti R. B lymphocytes and Epstein-Barr virus: the lesson of post-transplant lymphoproliferative disorders. Autoimmun Rev. 2007. 7:96–101.
2. Borhani AA, Hosseinzadeh K, Almusa O, Furlan A, Nalesnik M. Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics. 2009. 29:981–1000. discussion 1000-1002.
3. Chan SM, Hutnik CM, Heathcote JG, Orton RB, Banerjee D. Iris lymphoma in a pediatric cardiac transplant recipient: clinicopathologic findings. Ophthalmology. 2000. 107:1479–1482.
4. Cho AS, Holland GN, Glasgow BJ, Isenberg SJ, George BL, McDiarmid SV. Ocular involvement in patients with posttransplant lymphoproliferative disorder. Arch Ophthalmol. 2001. 119:183–189.
5. Cook T, Grostern RJ, Barney NP, Mills MD, Judd R, Albert DM. Posttransplantation lymphoproliferative disorder initially seen as iris mass and uveitis. Arch Ophthalmol. 2001. 119:768–770.
6. Fujita S, Fujikawa T, Mekeel K, Gonzalez R, Langham MR Jr, Foley DP, et al. Localized intraocular posttransplant lymphoproliferative disorder after pediatric liver transplantation. Transplantation. 2006. 81:493–495.
7. Pickhardt PJ, Siegel MJ, Hayashi RJ, Kelly M. Posttransplantation lymphoproliferative disorder in children: clinical, histopathologic, and imaging features. Radiology. 2000. 217:16–25.
8. Loevner LA, Karpati RL, Kumar P, Yousem DM, Hsu W, Montone KT. Posttransplantation lymphoproliferative disorder of the head and neck: imaging features in seven adults. Radiology. 2000. 216:363–369.
9. Cockfield SM. Identifying the patient at risk for post-transplant lymphoproliferative disorder. Transpl Infect Dis. 2001. 3:70–78.
10. Pickhardt PJ, Wippold FJ 2nd. Neuroimaging in posttransplantation lymphoproliferative disorder. AJR Am J Roentgenol. 1999. 172:1117–1121.
11. Mahajan A, Crum A, Johnson MH, Materin MA. Ocular neoplastic disease. Semin Ultrasound CT MR. 2011. 32:28–37.
12. Mancuso AA, Smith MF, Bhatt D, Verbist BM. Mancuso AA, Hanafee WN, Verbist BM, Hermans R, editors. Eye: intraocular neoplastic masses and vascular malformations. Head and Neck Radiology. 2011. Philadelphia: Williams & Wilkins;90–104.
13. Cunnane ME, Sepahdari A, Gardiner M, Mafee M. Som PM, Curtin HD, editors. Pathology of the eye and orbit. Head and Neck Imaging. 2011. 5th ed. Philadelphia: Mosby;602–639.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download