Abstract
Accurate prediction of cancer prognosis before the start of treatment is important since these predictions often affect the choice of treatment. Prognosis is usually based on anatomical staging and other clinical factors. However, the conventional system is not sufficient to accurately and reliably determine prognosis. Metabolic parameters measured by 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) have the potential to provide valuable information regarding prognosis and treatment response evaluation in cancer patients. Among these parameters, volume-based PET parameters such as metabolic tumor volume and total lesion glycolysis are especially promising. However, the measurement of these parameters is significantly affected by the imaging methodology and specific image characteristics, and a standard method for these parameters has not been established. This review introduces volume-based PET parameters as potential prognostic indicators, and highlights methodological considerations for measurement, potential implications, and prospects for further studies.
Accurate prediction of prognosis is important for the planning of effective treatment regarding cancer patients. However, due to the limitations of the conventional system alone, there is a need for more accurate and reliable methods of determining prognosis (1-5). In order to accomplish this, all the characteristics of a tumor, including biological, molecular, and clinical features, should be incorporated into the estimation of the patient's prognosis.
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) has become a standard imaging method for the staging, restaging, and monitoring of treatment response in a variety of tumors. This modality has an advantage over conventional imaging modalities in that it enables quantification of the metabolic activity of a tumor. Quantified metabolic activity can provide valuable information to help prognosticate and assess treatment response in clinical oncology. The standardized uptake value (SUV) is a commonly used parameter for (semi)-quantitative analysis of PET images, and is calculated either pixel-wise or over a region of interest as the ratio of tissue radioactivity concentration and the injected dose adjusted by body weight (6). The maximum SUV (SUVmax) is obtained for a 1-pixel region of interest (ROI) corresponding to the maximum pixel value in the tumor. This is a frequently used parameter because it provides an observer-independent measurement. Mean SUV (SUVmean), another common parameter, is the mean value of metabolic activity in a chosen region. However, SUVmax does not necessarily represent the total tumor activity for the whole tumor mass, because a single pixel may not be representative of nonhomogeneous overall tumor uptake. Peak SUV (SUVpeak), which is the average value within a small, fixed-size ROI in the tumor, is a more robust alternative to SUVmax. However, this measure shares many limitations with the SUVmax, and, more importantly, the ideal size and shape of the ROI has not yet been established (7). Although SUVmean may be more suitable for representing whole tumor activity, it is subjective and prone to observer variability (6).
Volume-based PET parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) have been developed (8) to measure metabolic activity in an entire tumor mass. These parameters are intended to measure global changes in tumor glycolysis. MTV is a volumetric measurement of tumor cells with high glycolytic activity, while TLG is defined as the product of the SUV and the lesion volume. Although these parameters are well-described in the literature, using these parameters in routine clinical practice is not easy because their measurement requires a considerable amount of time and effort. However, with the development of software capable of automated volume-of-interest (VOI) assessments, volume-based metabolic parameters have become easily available quantitative PET indices (9). Current clinical oncology guidelines do not yet reflect this advance, and do not include MTV measurements or TLG in characterizing the response to the treatment (8). However, these parameters have the potential to become a useful index for assessing therapeutic response by quantifying the global change in tumor burden during or after treatment. In addition, these parameters can also be used for predicting patient prognosis.
In this review, we focus on methodological considerations of the measurement of MTV and TLG, the potential usefulness of these parameters in assessing therapeutic response and predicting patient prognosis, and compare these parameters to determine performance.
Careful optimization of tumor delineation methods and image characteristics are important for obtaining accurate and reproducible volume-based PET parameters. Various methods for delineating tumor boundaries based on PET images have been reported, including purely manual and automatic methods, as well as several semiautomatic methods (10-14). In the manual method, tumor boundaries are manually drawn by a nuclear medicine physician, radiologist, or radiation oncologist, and the volume of that region is calculated. Manually drawing tumor boundaries can lead to a large variation in tumor volume because the determination of the tumor boundary depends on both the experience of the physician and the contouring protocol used (15). Automatic or semiautomatic methods can be used to delineate tumor boundaries using an isocontour threshold in which all contiguous voxels with values above a chosen threshold are included. An advantage of this method is that inter- and intra-observer variation in tumor volume delineation is reduced. Figure 1 shows a representative example using automatically generated VOIs to measure the MTV of a primary tumor. Automatic and semiautomatic methods with various threshold values have been reported in previous studies. The fixed threshold method is a widely used and simple technique that applies a threshold based on a percentage (typically, 41-70%) of SUVmax within the tumor. Results of previous work have shown that the ideal percentage for the threshold SUV to most accurately reflect the pathological tumor size is inversely proportional to the SUVmax of the tumor (16, 17). However, the fixed threshold method for measuring tumor volume has several limitations. First, the tumor volume may be significantly affected by noise because the value of SUVmax depends on the amount of noise present (6). A second limitation of this method is that if the percentage used for the fixed threshold is too low, the tumor volume may erroneously include a significant proportion of the background (6). To overcome these problems, the isocontour value can be based on the difference between tumor activity and background activity (13). This adaptive threshold method is similar to the fixed threshold method. However, this method adjusts the threshold value relative to the local average background, thereby correcting for the contrast between the tumor and local background (18). The contrast-oriented method applies correction based on the mean of 70% SUVmax and the background activity for various sphere sizes (18). The absolute SUV method adopts a certain SUV value (e.g., SUV 2.5) that would properly differentiate between benign and malignant lesions as the absolute threshold value for delineating the tumor (14, 19). The problem with this method is that the absolute threshold value may be somewhat arbitrary.
Mediastinal blood pool activity (9, 20, 21) and liver activity (9, 22) can also be used as threshold values. In addition to these methods, there are many other types of automatic and semiautomatic methods, such as the background-subtracted relative threshold level method (18), the gradient-based watershed segmentation method (18), and the fuzzy locally adaptive Bayesian method (23).
It should be noted that there is still no consensus on a standard method for tumor delineation. It is important to use MTV and TLG because the measurement of volume-based PET parameters is significantly affected by the tumor delineation method (21, 23, 24). A recent study reported that all tumor delineation methods demonstrated a much larger variation in measured metabolic tumor volume (< 29%) compared to SUV (< 11%) when image characteristics and radiotracers were varied (24). In another study, large differences were observed between methods (from -140 to 50% of tumor volume) (23). A recent phantom study showed that a contrast-oriented method provided the most accurate results, on average, over all simulated conditions (18). Another study investigated the impact of tumor delineation methods in patients with esophageal cancer and suggested that the fuzzy locally adaptive Bayesian method may be the most useful method (23). However, while many methods have been proposed for the determination of an optimal threshold in regards to tumor delineation (2, 9, 12, 18-23), a standard method has not yet been established for clinical use (9). There is some controversy regarding whether the value of volume-based PET parameters in clinical use is affected by the tumor delineation method used. Although the tumor delineation method used had a significant quantitative impact on the absolute value of the PET parameters, the clinical value of the PET parameters may not be affected. In an earlier study evaluating the use of MTV and TLG for predicting recurrence-free survival (RFS) and overall survival (OS) in patients with non-small cell lung cancer, patients with smaller MTV and lower TLG showed longer RFS and OS regardless of the threshold value used (25). A similar result was demonstrated in a recent study of patients with tonsil cancer, in which the value of the metabolic parameters in predicting overall survival was not significantly affected by the selected threshold value (9).
The accuracy of PET-based automatic or semiautomatic delineation methods can be affected by many factors, including image resolution and reconstruction settings (10, 11, 26). Previous studies have reported that the performance of various automatic or semiautomatic tumor delineation methods depends on both image resolution and contrast (18, 24). In a recent study that assessed the test-retest variability of tumor delineation methods according to the effects of several image characteristics, the variability of both metabolic tumor volume and SUV varied with radiotracer, image contrast, and image resolution. In that study, there was substantial variability (≤ 94%) in the measured tumor volume when the image resolution or contrast was changed (24). The test-retest variability of the median metabolic volume according to the image characteristics ranged from 8.3% to 23% and from 7.4% to 29% for 18F-FDG and 18F-FLT, respectively. The difference in the test-retest variability of the metabolic tumor volume was larger than that of SUV (24). Another recent study reported that the absolute quantitative value obtained through the tumor delineation method depends on the variation in the tumor-to-background ratio (TBR), image resolution and image noise level, and to a lesser extent, on the number of iterations performed during image reconstruction (18).
The partial-volume effect (PVE) may affect MTV or TLG (27), but PVE correction may not be sufficient to improve the predictive or prognostic value of these parameters. In a study of 50 patients with esophageal cancer, PVE correction had a significant quantitative impact on the absolute values of the investigated parameters. However, the clinical value of MTV and TLG in predicting the response and OS was not significantly different before and after correction (27).
Breathing motion can also affect the measurement of metabolic tumor volume, and the use of a respiratory gating system allows for a more accurate measurement of tumor volume based on PET images. Measurements of tumor volume in the gated mode have been reported to be reduced up to 34% compared with the non-gated mode. However, TLGs measured in gated and non-gated modes showed consistent results, which can be explained by the reduction in tumor volume being accompanied by an increase in the intensity of the 18F-FDG signal per voxel (28).
Metabolic tumor volume can either be derived from images of the glucose metabolic rate (generated using Patlak analysis) or from SUV images (29). While SUV requires a static scan, images of the glucose metabolic rate can be generated from dynamic scans. Metabolic volumes derived from images of the glucose metabolic rate are generally smaller than those derived from SUV images (29). Metabolic volumes derived from SUV images rather than from the images of glucose metabolic rate tend to produce more extreme values, except when gradient-based methods are used. Median measured metabolic volumes derived from SUV images were larger than those derived from dynamic images (up to a 59% difference) when using a fixed percentage threshold method (29).
Although there has recently been a dramatic increase in the number of studies investigating the value of volume-based metabolic parameters, the extent to which volume-based PET parameters can be significantly influenced by factors such as the tumor delineation method, image resolution, contrast, noise, radiotracer, or reconstruction settings has not been definitively determined. However, it is clear that careful optimization of tumor delineation methods and image characteristics are important for the reproducible measurement of volume-based PET parameters, as this can be affected by the factors mentioned above. In particular, the tumor delineation method has the most significant quantitative impact on the absolute value of these PET parameters, and it is essential to use consistent imaging characteristics and tumor delineation methods in measuring volume-based PET parameters, particularly for longitudinal studies.
Metabolic tumor volume and TLG are potential prognostic indicators for various cancers. The baseline metabolic tumor burdens expressed by these parameters have been shown to be closely associated with patient prognosis. Table 1 summarizes the literature regarding the prediction of the clinical course before treatment using MTV or TLG. It has been reported that as the volume-based parameters of a tumor become smaller and lower, the prognosis for lung cancer improves (25, 30, 31). Similar results have been shown for head and neck cancers (9, 32-36), colorectal cancers (37, 38), esophageal cancers (23, 27), gynecological cancers (39, 40), and others (41-44). Figure 2 illustrates the survival curves according to PET parameters in tonsilar cancer (9). Both MTV and TLG discriminate between the two survival curves better than SUVmax, indicating that these values may be a useful quantitative index for disease prognosis prior to treatment. Furthermore, the combination of volume-based PET parameters with clinical prognostic factors may significantly improve prognostic stratification in cancer patients. For example, in a study of advanced nasopharyngeal cancer patients, there was a stepwise decrease in local and distant control rates according to a scoring system formulated by combining PET parameters and traditional prognostic factors (32).
The parameters we have described can also be useful in assessing therapeutic response. Currently, the Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1, which is primarily based on changes in the longest dimension of a tumor, is the standard method for assessing the treatment response in patients with solid tumors (45). However, change in the longest dimension is insufficient to accurately reflect change in tumor burden. In addition, these criteria do not reflect the functional and metabolic changes that may occur with targeted chemotherapy (45). In contrast to conventional chemotherapy, targeted therapies usually do not lead to rapid tumor cell death. Targeted therapies interfere with the tumor signaling pathways, thus inhibiting tumor cell proliferation. Therefore, the previous morphological approach is not appropriate for assessing the early response to targeted therapies. Metabolic changes as measured by PET may supplement conventional methods in assessing treatment response, thus addressing some of their limitations. Table 2 summarizes the literature on evaluating therapy response using MTV or TLG. Changes in MTV and TLG between pre- and post-treatment scans may be a useful index in the prediction of therapeutic response for various cancers (46). One previous study investigated the relationship between chemotherapy and 18F-FDG uptake in a mouse model of lymphoma, and found that 18F-FDG uptake reflected the dose-response relationship of chemotherapy and that TLG was the best parameter for dose-related response assessment (47). These parameters have also been reported to be useful for assessing tumor down-staging and determining the percentage of residual tumor following neoadjuvant treatment, which could potentially assist in treatment planning for patients with rectal cancer (48). In a prospective study of osteosarcoma patients, MTV and TLG were significantly different after therapy between good and poor responders. These parameters had good sensitivity, specificity, positive predictive value, and negative predictive value for predicting histological responses to chemotherapy (49). In another study comparing response evaluation using volume-based PET parameters to a clinical response evaluation based on RECIST and WHO clinical criteria in patients with esophageal cancer, a decrease in MTV and TLG between baseline and post-treatment scans was the better predictor of histopathologic response and survival than a decrease in clinical response (50). However, while there have been many positive results in previous studies supporting the usefulness of MTV and TLG (48-51), it is difficult to prove that 18F-FDG PET is superior to conventional imaging techniques in assessing therapy response. For example, a recently published study reported that the correlations of volume-based PET parameters and OS are inferior to those of CT-derived volume parameters in patients with malignant pleural mesothelioma receiving continual pemetrexed- and platin-based treatment (41).
One major shortcoming of using PET parameters in assessing the response to therapy is that 18F-FDG PET may not differentiate radiation-related inflammation from the residual tumor in patients who received either radiotherapy or chemoradiotherapy (51). A recent prospective study reported that metabolic parameters including TLG were not associated with the prognosis of rectal cancer patients who had received neoadjuvant chemoradiotherapy (52). From this, it can be extrapolated that radiation-related inflammation may be a major obstacle limiting the use of PET parameters in the assessment of radiotherapy response.
Although many previous studies have investigated the usefulness of volume-based PET parameters in predicting prognosis and evaluating treatment response and have shown that MTV and TLG can be useful indicators in a variety of tumors and in various clinical settings, most of these studies are retrospective and use a relatively small number of subjects. Therefore, further well-designed large-scale prospective studies are needed in order to confirm the clinical value of these parameters.
Radiotherapy planning is another context in which volume-based PET parameters may be useful (14). When comparing PET-based tumor volume to CT-based tumor volume in intensity-modulated radiotherapy for head-and-neck cancer, one study found that the PET-based tumor volume was smaller, the same size, or larger than the CT-based tumor volume in 75%, 8%, and 18% cases, respectively. In the same study, 75% of study subjects received at least 95% of the prescribed dose obtained using CT on the PET-based tumor volume (14). In another recent study (53) in which 18F-FDG PET/CT scans were performed during radiation therapy after every seventh fraction, the volume of each lesion was measured by an experienced nuclear physician using visual delineation with a 40% SUVmax fixed threshold and a semi-automatic adaptive threshold method. An average decrease in SUVmax of 50% was observed around 40-45 Gy (i.e., during week 5 of radiation therapy [RT]). The three delineation methods yielded consistent volume measurements before RT and during the first week of radiation therapy; however, as the course of radiation therapy continued, manual delineation appeared to be more reliable (53).
Standard values for comparing MTV and TLG have not been established. In particular, the question regarding which parameter is superior for predicting outcomes and assessing treatment response still remains (9, 23). There is a great deal of evidence to support the hypothesis that TLG, a combination of SUV and MTV, is the ideal metabolic parameter for tumor burden as it simultaneously represents the degree of 18F-FDG uptake and the size of the metabolically-active tumor mass (9, 30, 32, 35). It has also been reported that the optimal PET-based estimate for the total tumor burden is the sum of TLG over all lesions (54). In addition, in previous studies, TLG was identified as a significant independent predictor of clinical course, associated with OS and RFS, while MTV was not a significant prognostic factor (9, 30, 32, 35). However, a conflicting study reported that only a change in MTV identified complete responders after neoadjuvant CCRT in patients with esophageal cancer (51). The comparison between TLG and MTV for prognostic prediction and response evaluation remains controversial.
In determining whether MTV and TLG are significant and independent prognostic factors through multiple regression model analysis, we note that multicollinearity, the statistical phenomenon in which variables are strongly correlated with each other, is a substantial barrier to easy interpretation. When two variables are strongly correlated, like MTV and TLG, it is difficult to develop reliable calculations regarding the individual variables (55). One possible alternative to comparing the prognostic value of these parameters is to analyze MTV and TLG in a separate MTV or TLG model, then assess the discriminative performance of each model.
Metabolic tumor volume and TLG are generally highly correlated with tumor volume as measured by computed tomography (CT). It has been reported that there is a high correlation between MTV and CT-based volume for lesions greater than 5 cm3 in size (54). It has also been reported that volume-based PET parameters are positively correlated with T-stage, specifically in the setting of primary nasopharyngeal carcinoma (56). In addition, recently reported data on the relationship between viral load and PET parameters demonstrated that of the tested parameters, total TLG had the highest correlation with Epstein-Barr virus deoxyribonucleic acid, a known prognostic factor in patients with nasopharyngeal carcinoma (57).
Correlations between diffusion-weighted magnetic resonance imaging parameters and volume-based PET parameters have been reported (58). There is a significant positive correlation between the total diffusivity index and TLG (58). This significant correlation between parameters suggests an association between tumor cellularity and metabolic activity (58). Another recent study also reported a significant positive correlation between choline concentration relative to water (Cho/W) as measured by proton magnetic resonance spectroscopy (H-MRS) and TLG (59). Elevated levels of Cho-containing compounds in tumors are related to membrane synthesis (60), and Cho/W is thought to be an index that may reflect cell proliferation (60). The results of that study indicate that volume-based PET parameters most likely indirectly reflect the proliferation of tumor cells.
As discussed above, MTV and TLG are both potential parameters for evaluating patient prognosis and assessing therapeutic response. However, the value of these parameters has not yet been established, and the evidence from previous retrospective studies using different methods for measurement is insufficient for validating these parameters for clinical practice. Therefore, large-scale prospective studies analyzing the effects of methodological factors important in measuring these parameters are needed in order to extend their use. Several important aspects should be considered in planning such studies. First, a tumor delineation method that can provide reliable and reproducible results should be selected, and determination of an optimal threshold may be the most crucial step in the process. Additionally, ease of measurement is also an important factor for methods that will see routine clinical use. Second, the total MTV or TLG of all tumor lesions, including primary tumors and metastasis, most likely reflects tumor burden more precisely than the MTV or TLG of primary tumor lesions. However, in most reported studies, MTV and TLG were only measured in prominent tumor sites with significantly increased FDG uptake. Only a few studies have demonstrated that the MTV of all malignant lesions is an independent predictor for progression and death (61, 62). The target tumor lesions for the measurement of volume-based PET parameters should be extended to the entire tumor burden. Third, the clinical impact of these parameters should be evaluated in further studies. In particular, well-designed studies are needed to establish whether volume-based PET parameters should affect the selection of the treatment plan. We expect that the number of studies of the use of volume-based PET parameters in clinical oncology will continue to increase.
The volume-based PET parameters MTV and TLG are useful indices of tumor burden. Although the role of these parameters has not yet been established, they are potentially useful parameters for the prognostication and evaluation of treatment response in cancer patients. In using volume-based PET parameters, careful optimization of tumor delineation methods and image characteristics is crucial, as these parameters can be significantly affected by the tumor delineation method, image resolution, contrast, noise, the radiotracer used, and the reconstruction settings. In particular, the tumor delineation method used is the most important factor affecting the measurement of these parameters. Many previous studies have demonstrated that these parameters are closely associated with patient prognosis and are related to other established prognostic factors. However, further large-scale prospective studies are needed in order to confirm the value of these parameters.
Figures and Tables
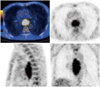 | Fig. 1Measurement of metabolic tumor volume in patients with esophageal cancer.
18F-fluorodeoxyglucose PET/CT images of 53-year-old male patient with esophageal cancer demonstrating measurement of metabolic tumor volume. Boundary of metabolically active tumor was automatically delineated using isocontour, defined as percentage of maximum SUV in tumor (40% in this image). VOI = volume of interest, SUV = standardized uptake values
|
 | Fig. 2Kaplan-Meier survival curves for overall survival using MTV (A), TLG (B), and SUVmax (C) in 69 patients with squamous cell carcinoma of tonsil.
MTV = metabolic tumor volume, TLG = total lesion glycolysis, SUVmax = maximum standardized uptake value
|
References
1. Takes RP, Rinaldo A, Silver CE, Piccirillo JF, Haigentz M Jr, Suárez C, et al. Future of the TNM classification and staging system in head and neck cancer. Head Neck. 2010. 32:1693–1711.
2. Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007. 69:328–333.
3. Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol. 2012. 4:128–134.
4. Walters TK, Zuckerman J, Nisbet-Smith A, Hudson E, Chia Y, Burke M. Fine needle aspiration biopsy in the diagnosis and management of fibroadenoma of the breast. Br J Surg. 1990. 77:1215–1217.
5. Chun YH, Kim SU, Park JY, Kim do Y, Han KH, Chon CY, et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer. 2011. 47:2568–2575.
6. Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007. 48:932–945.
7. Vanderhoek M, Perlman SB, Jeraj R. Impact of the definition of peak standardized uptake value on quantification of treatment response. J Nucl Med. 2012. 53:4–11.
8. Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging. The Visual Response Score and the Change in Total Lesion Glycolysis. Clin Positron Imaging. 1999. 2:159–171.
9. Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: Comparisons of volume-based metabolic parameters. Head Neck. 2012. [Epub ahead of print].
10. Geets X, Lee JA, Bol A, Lonneux M, Grégoire V. A gradient-based method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med Mol Imaging. 2007. 34:1427–1438.
11. Schaefer A, Kremp S, Hellwig D, Rübe C, Kirsch CM, Nestle U. A contrast-oriented algorithm for FDG-PET-based delineation of tumour volumes for the radiotherapy of lung cancer: derivation from phantom measurements and validation in patient data. Eur J Nucl Med Mol Imaging. 2008. 35:1989–1999.
12. van Dalen JA, Hoffmann AL, Dicken V, Vogel WV, Wiering B, Ruers TJ, et al. A novel iterative method for lesion delineation and volumetric quantification with FDG PET. Nucl Med Commun. 2007. 28:485–493.
13. Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004. 45:1519–1527.
14. Paulino AC, Koshy M, Howell R, Schuster D, Davis LW. Comparison of CT- and FDG-PET-defined gross tumor volume in intensity-modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005. 61:1385–1392.
15. MacManus M, Nestle U, Rosenzweig KE, Carrio I, Messa C, Belohlavek O, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006-2007. Radiother Oncol. 2009. 91:85–94.
16. Zhong X, Yu J, Zhang B, Mu D, Zhang W, Li D, et al. Using 18F-fluorodeoxyglucose positron emission tomography to estimate the length of gross tumor in patients with squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2009. 73:136–141.
17. Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010. 17:115–122.
18. Cheebsumon P, Yaqub M, van Velden FH, Hoekstra OS, Lammertsma AA, Boellaard R. Impact of [18F]FDG PET imaging parameters on automatic tumour delineation: need for improved tumour delineation methodology. Eur J Nucl Med Mol Imaging. 2011. 38:2136–2144.
19. Meng X, Sun X, Mu D, Xing L, Ma L, Zhang B, et al. Noninvasive evaluation of microscopic tumor extensions using standardized uptake value and metabolic tumor volume in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012. 82:960–966.
20. Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007. 25:571–578.
21. Le Roux PY, Gastinne T, Le Gouill S, Nowak E, Bodet-Milin C, Querellou S, et al. Prognostic value of interim FDG PET/CT in Hodgkin's lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging. 2011. 38:1064–1071.
22. de Jong PA, van Ufford HM, Baarslag HJ, de Haas MJ, Wittebol SH, Quekel LG, et al. CT and 18F-FDG PET for noninvasive detection of splenic involvement in patients with malignant lymphoma. AJR Am J Roentgenol. 2009. 192:745–753.
23. Hatt M, Visvikis D, Albarghach NM, Tixier F, Pradier O, Cheze-le Rest C. Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumour delineation methodology. Eur J Nucl Med Mol Imaging. 2011. 38:1191–1202.
24. Cheebsumon P, van Velden FH, Yaqub M, Frings V, de Langen AJ, Hoekstra OS, et al. Effects of image characteristics on performance of tumor delineation methods: a test-retest assessment. J Nucl Med. 2011. 52:1550–1558.
25. Kim K, Kim SJ, Kim IJ, Kim YS, Pak K, Kim H. Prognostic value of volumetric parameters measured by F-18 FDG PET/CT in surgically resected non-small-cell lung cancer. Nucl Med Commun. 2012. 33:613–620.
26. Daisne JF, Sibomana M, Bol A, Doumont T, Lonneux M, Grégoire V. Tri-dimensional automatic segmentation of PET volumes based on measured source-to-background ratios: influence of reconstruction algorithms. Radiother Oncol. 2003. 69:247–250.
27. Hatt M, Le Pogam A, Visvikis D, Pradier O, Cheze Le Rest C. Impact of partial-volume effect correction on the predictive and prognostic value of baseline 18F-FDG PET images in esophageal cancer. J Nucl Med. 2012. 53:12–20.
28. Nehmeh SA, Erdi YE, Ling CC, Rosenzweig KE, Schoder H, Larson SM, et al. Effect of respiratory gating on quantifying PET images of lung cancer. J Nucl Med. 2002. 43:876–881.
29. Cheebsumon P, van Velden FH, Yaqub M, Hoekstra CJ, Velasquez LM, Hayes W, et al. Measurement of metabolic tumor volume: static versus dynamic FDG scans. EJNMMI Res. 2011. 1:35.
30. Arslan N, Tuncel M, Kuzhan O, Alagoz E, Budakoglu B, Ozet A, et al. Evaluation of outcome prediction and disease extension by quantitative 2-deoxy-2-18F fluoro-D-glucose with positron emission tomography in patients with small cell lung cancer. Ann Nucl Med. 2011. 25:406–413.
31. Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012. 39:27–38.
32. Chan SC, Chang JT, Lin CY, Ng SH, Wang HM, Liao CT, et al. Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for different survival endpoints and impact on prognostic stratification. Nucl Med Commun. 2011. 32:989–996.
33. Chu KP, Murphy JD, La TH, Krakow TE, Iagaru A, Graves EE, et al. Prognostic value of metabolic tumor volume and velocity in predicting head-and-neck cancer outcomes. Int J Radiat Oncol Biol Phys. 2012. 83:1521–1527.
34. La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009. 74:1335–1341.
35. Xie P, Yue JB, Zhao HX, Sun XD, Kong L, Fu Z, et al. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2010. 136:883–889.
36. Choi KH, Yoo IR, Han EJ, Kim YS, et al. Prognostic Value of Metabolic Tumor Volume Measured by 18F-FDG PET/CT in Locally Advanced Head and Neck Squamous Cell Carcinomas Treated by Surgery. Nucl Med Mol Imaging. 2011. 45:43–51.
37. Guillem JG, Moore HG, Akhurst T, Klimstra DS, Ruo L, Mazumdar M, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J Am Coll Surg. 2004. 199:1–7.
38. Gulec SA, Suthar RR, Barot TC, Pennington K. The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing 90Y selective internal radiation therapy plus chemotherapy. Eur J Nucl Med Mol Imaging. 2011. 38:1289–1295.
39. Chung HH, Kwon HW, Kang KW, Park NH, Song YS, Chung JK, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann Surg Oncol. 2012. 19:1966–1972.
40. Kim BS, Kim IJ, Kim SJ, Nam NH, Park KJ, Kim K, et al. The Prognostic value of the metabolic tumor volume in FIGO stage IA to IIB cervical cancer for tumor recurrence: measured by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2011. 45:36–42.
41. Schaefer NG, Veit-Haibach P, Soyka JD, Steinert HC, Stahel RA. Continued pemetrexed and platin-based chemotherapy in patients with malignant pleural mesothelioma (MPM): value of 18F-FDG-PET/CT. Eur J Radiol. 2012. 81:e19–e25.
42. Costelloe CM, Macapinlac HA, Madewell JE, Fitzgerald NE, Mawlawi OR, Rohren EM, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009. 50:340–347.
43. Yoo J, Choi JY, Lee KT, Heo JS, Park SB, Moon SH, et al. Prognostic significance of volume-based metabolic parameters by 18F-FDG PET/CT in gallbladder carcinoma. Nucl Med Mol Imaging. 2012. 46:201–206.
44. Lee HY, Hyun SH, Lee KS, Kim BT, Kim J, Shim YM, et al. Volume-based parameter of 18F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol. 2010. 17:2787–2794.
45. Kang H, Lee HY, Lee KS, Kim JH. Imaging-based tumor treatment response evaluation: review of conventional, new, and emerging concepts. Korean J Radiol. 2012. 13:371–390.
46. Kiyohara S, Nagamachi S, Wakamatsu H, Nishii R, Fujita S, Futami S, et al. [Usefulness of metabolic volume and total lesion glycolysis for predicting therapeutic response in cancer therapy by 18F-FDG PET/CT]. Kaku Igaku. 2010. 47:453–461.
47. Brepoels L, De Saint-Hubert M, Stroobants S, Verhoef G, Balzarini J, Mortelmans L, et al. Dose-response relationship in cyclophosphamide-treated B-cell lymphoma xenografts monitored with [18F]FDG PET. Eur J Nucl Med Mol Imaging. 2010. 37:1688–1695.
48. Melton GB, Lavely WC, Jacene HA, Schulick RD, Choti MA, Wahl RL, et al. Efficacy of preoperative combined 18-fluorodeoxyglucose positron emission tomography and computed tomography for assessing primary rectal cancer response to neoadjuvant therapy. J Gastrointest Surg. 2007. 11:961–969. discussion 969.
49. Im HJ, Kim TS, Park SY, Min HS, Kim JH, Kang HG, et al. Prediction of tumour necrosis fractions using metabolic and volumetric 18F-FDG PET/CT indices, after one course and at the completion of neoadjuvant chemotherapy, in children and young adults with osteosarcoma. Eur J Nucl Med Mol Imaging. 2012. 39:39–49.
50. Roedl JB, Colen RR, Holalkere NS, Fischman AJ, Choi NC, Blake MA. Adenocarcinomas of the esophagus: response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET-CT. Comparison to histopathologic and clinical response evaluation. Radiother Oncol. 2008. 89:278–286.
51. Arslan N, Miller TR, Dehdashti F, Battafarano RJ, Siegel BA. Evaluation of response to neoadjuvant therapy by quantitative 2-deoxy-2-[18F]fluoro-D-glucose with positron emission tomography in patients with esophageal cancer. Mol Imaging Biol. 2002. 4:301–310.
52. Ruby JA, Leibold T, Akhurst TJ, Shia J, Saltz LB, Mazumdar M, et al. FDG-PET assessment of rectal cancer response to neoadjuvant chemoradiotherapy is not associated with long-term prognosis: a prospective evaluation. Dis Colon Rectum. 2012. 55:378–386.
53. Edet-Sanson A, Dubray B, Doyeux K, Back A, Hapdey S, Modzelewski R, et al. Serial assessment of FDG-PET FDG uptake and functional volume during radiotherapy (RT) in patients with non-small cell lung cancer (NSCLC). Radiother Oncol. 2012. 102:251–257.
54. Akhurst T, Ng V V, Larson SM, O'Donoghue JA, O'Neel J, Erdi Y, et al. Tumor Burden Assessment with Positron Emission Tomography with. Clin Positron Imaging. 2000. 3:57–65.
55. O'BRIEN RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007. 41:673–690.
56. Chan WK, Mak HK, Huang B, Yeung DW, Kwong DL, Khong PL. Nasopharyngeal carcinoma: relationship between 18F-FDG PET-CT maximum standardized uptake value, metabolic tumour volume and total lesion glycolysis and TNM classification. Nucl Med Commun. 2010. 31:206–210.
57. Chang KP, Tsang NM, Liao CT, Hsu CL, Chung MJ, Lo CW, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med. 2012. 53:21–28.
58. Gu J, Khong PL, Wang S, Chan Q, Law W, Zhang J. Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDG-PET/CT. Mol Imaging Biol. 2011. 13:1020–1028.
59. Jansen JF, Schöder H, Lee NY, Stambuk HE, Wang Y, Fury MG, et al. Tumor metabolism and perfusion in head and neck squamous cell carcinoma: pretreatment multimodality imaging with 1H magnetic resonance spectroscopy, dynamic contrast-enhanced MRI, and [18F]FDG-PET. Int J Radiat Oncol Biol Phys. 2012. 82:299–307.
60. Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999. 59:80–84.
61. Oh JR, Seo JH, Chong A, Min JJ, Song HC, Kim YC, et al. Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012. 39:925–935.
62. Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer. 2011. 73:332–337.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download