Abstract
Objective
To assess the technical success, ability to eat, complications and clinical outcomes of patients with self-expandable metal stent (SEMS) placed for malignant upper gastrointestinal (GI) obstruction.
Materials and Methods
Data was collected retrospectively on patients who underwent SEMS placement for palliation of malignant upper GI obstruction by reviewing hospital charts from June 1998 to May 2011. Main outcome measurements were technical success, gastric outlet obstruction scoring system (GOOSS) score before and after treatment, complications, and survival.
Results
A total of 82 patients underwent SEMS placement with malignant upper GI obstruction. The initial SEMS placement was successful in 77 patients (93.9%). The mean GOOSS score was 0.56 before stenting and 1.92 (p < 0.001) after treatment. Complications arose in 12 patients (14.6%): stent migration in 1 patient (1.2%), perforation in 1 (1.2%), and obstruction of stent due to tumor ingrowth in 10 (12.2%). The median survival time after stenting was 52 days (6-445).
Malignant upper gastrointestinal (GI) obstruction is a late complication of advanced esophageal, gastric, duodenal, or periampullary malignancies (including carcinoma of the head of the pancreas, distal bile duct, or ampulla of Vater). It leads to malnutrition and a decrease in the quality of life. Patients are at risk of aspiration and pneumonia (1). Obstruction greatly diminishes the quality of life in these patients who have a limited life expectancy (2). self-expandable metal stent (SEMS) are being increasingly used as a safe and effective alternative to surgery or repetitive endoscopic procedure to improve the quality of life for patients with upper GI diseases and disorder. Patients who are not surgical candidates can benefit from upper GI stenting to alleviate obstruction, provided there is no perforation or peritonitis. Recent reports (3-9) describe SEMS placement as a desirable alternative to bypass surgery, which is associated with significant risks of morbidity and mortality. Accordingly, we reviewed our experience with SEMS placed for the palliation of malignant upper GI obstruction with the aim of assessing the feasibility, safety, and efficacy of this treatment.
Patients who underwent SEMS placement from June 1998 to May 2011 for palliation of malignant upper GI obstruction were identified by reviewing hospital records, clinical notes and endoscopy procedure reports. All patients were deemed to be poor candidates for primary surgical intervention based on advanced disease or comorbidities. Data were obtained from hospital records, clinical notes, endoscopy procedure reports, and follow up clinical visits after stenting. Data collected included patient demographics, indication for stenting including the type of malignancy and the site of the malignant obstruction, the gastric outlet obstruction scoring system (GOOSS) score before and after stenting, the type of enteral stent, the length of hospital stay, complications, and survival.
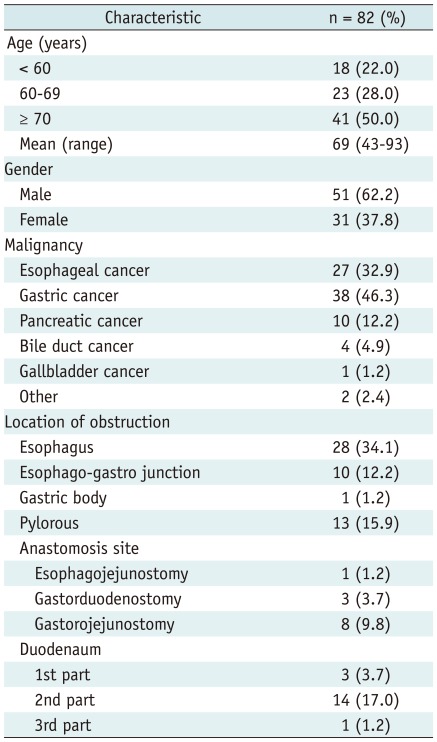
A total of 82 patients underwent SEMS placement with malignant upper GI obstruction between June 1998 and May 2011. The characteristics of the 82 patients are summarized in Table 1. Mean patient age was 69 years (range, 43-93 years) and 51 patients were male. The obstruction of the upper GI tract was caused by esophageal cancer in 27 (32.9%) patients, gastric cancer in 38 (46.3%), pancreatic cancer in 10 (12.2%), bile duct cancer in 4 (4.9%), gallbladder cancer in 1 (1.2%), and other malignancies in 2 (2.4%). The site of obstruction was the esophagus in 28 patients (34.1%), the esophago-gastro junction (EGJ) in 10 (12.2%), the gastric body in 1 (1.2%), the pylorus in 13 (15.9%), the anastomosis side in 12 (14.6%) (eophagojejunostomy in 1, gastroduodenostomy in 3, gastrojejunostomy in 8), and the duodenum in 18 (22.0%) patients. Seven of the 27 patients with esophageal cancer had a bronchoesophageal fistula. Six of these 7 patients received chemoradiation therapy before stent placement. Fifty of the 82 patients could not tolerate any kind of food before treatment.
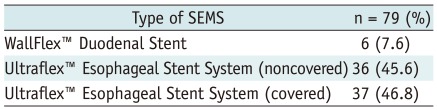
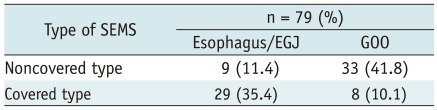
Patients underwent endoscopy with fluoroscopic guidance to delineate the length of obstruction. Placement of clips was performed in selected patients at the discretion of the endoscopist to mark the proximal and distal end of a stricture. SEMS placement was performed over a guide wire under endoscopic and/or fluoroscopic guidance. The stent used in all patients for the June 1998-February 2010 period was the Ultraflex™ Esophageal Stent System (Boston Scientific Co., Natick, MA, USA). We extended the delivery system when we placed the stent for gastroduodenal obstruction. Also, we introduced the WallFlex™ Duodenal Stent (Boston Scientific Co., Natick, MA, USA) from February 2010 for gastric outlet and duodenal obstruction. Ultraflex™ Esophageal Stent System is available in diameters of 17 and 22 mm (covered type), 18 mm (noncovered type) and lengths of 10, 12, and 15 cm (covered type), and 7, 10 and 15 cm (noncovered type). WallFlex™ Duodenal Stent is available in a diameter of 22 mm, and lengths of 60, 90 and 120 mm. The attending endoscopist determined the diameter and length of SEMS according to the disease state of the patient. Basically, covered SEMS was used for the obstruction of esophagus and EGJ. In contrast, noncovered SEMS was used for the gastric outlet obstruction (GOO). Technical success was defined as correct placement of the stent across the stricture, with patency.
Perforation, stent migration, and hemorrhage were considered as early complications (< 72 h). Late complications (≥ 72 h) were migration, perforation, and tumor ingrowth. Patients were observed for 72 hours after stenting monitor for early complications. A clear liquid diet was allowed from the morning following the stenting, and the diet was advanced as tolerated. Clinical results and number of days from the time of stenting and discharge from the hospital were noted. We also recorded when a second stent was placed. If the patients could be discharged from the hospital, they were seen in the outpatient clinic of our hospital every 1-4 weeks.
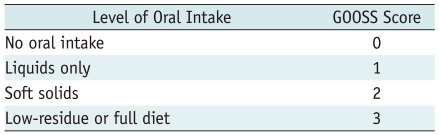
Data are shown as a number (%) for categorical variables or as mean (range) for continuous variables. The degree of dysphagia was assessed before and after stent placement by using an adaptation of GOOSS produced by Adler and Baron (10), with swallowing ability divided into 4 categories: 0, no oral intake; 1, liquids only; 2, soft solid; 3, low-residue or full diet (Table 2). Pre- and post-stenting GOOSS score were analyzed by using the Chi-square test. Cumulative patient survival was estimated by using the Kaplan-Meier method. The significance of difference in survival time after stent placement was determined by Log-rank test. A p value of less than 0.05 was considered to be statistically significant. Analysis was performed using SSPS software version 11.0J (SPSS Inc., Chicago, IL, USA).
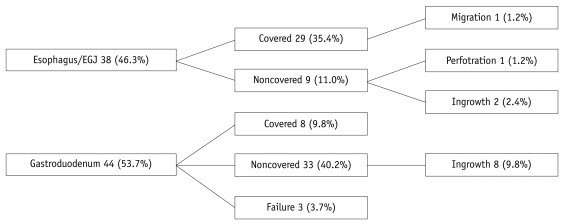
The initial stent placement was successful in 77 of 82 patients (93.9%). Five patients had technical failure at the first treatment; in 3 patients the delivery device did not pass the malignant stricture, in 2 the SEMS was placed at an inadequate position. These two patients in whom SEMS were placed at the inadequate position underwent a second stent placement successfully. Therefore, technical success was achieved finally in 79 patients (96.3%). Of the 79 patients in which a SEMS was placed, 6 (7.6%) were performed with the TTS delivery technique and 73 (92.4%) with the non-TTS technique. The detail of the type of SEMS and presence or absence of cover according to the site of obstruction are shown in Tables 3, 4.
All patients were followed until May 2011 or death. Thirty-five patients were able to be discharged from the hospital. Of the 79 patients with successful SEMS placement, 77 were able to tolerate oral intake without obstructive symptoms, giving a clinical success rate of 97.5%. Two patients who could not tolerate an oral intake after successful stent placement appeared to have peritoneal dissemination.
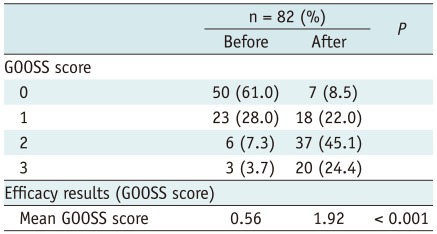
At baseline 50 patients (61%) were incapable of any oral intake (GOOSS score = 0) , and 23 (28%) could tolerate liquids only (GOOSS score = 1). After SEMS placement, 57 patients (69.5%) had been able to resume solid food (GOOSS score = 2-3). GOOSS score showed a statistically significant improvement before (mean, 0.56) and after (mean, 1.92) (p < 0.001) stent placement (Table 5). Thirty-five of 82 patients were able to discharge after SEMS placement, with a median time from SEMS placement to discharge of 18 days (range, 1-76). The median length of the hospital stay of patients who were not able to discharge was 32 days (range, 7-100). The mean length of hospital stay of the patient who was possible for discharge and the patient who was impossible were 23 days and 36.9 days (p < 0.01), respectively.
There was no early complication (< 72 h). The late complications (≥ 72 h) arose in 12 patients (14.6%). Stent migration occurred in 1 patient (1.2%) on day 29, perforation in 1 (1.2%) with esophageal cancer after chemoradiation therapy on day 105, and obstruction of stent due to tumor ingrowth in 10 (12.2%). Eight of 10 patients with tumor ingrowth required second SEMS placement, and one of the other two was treated by Argon Plasma Coagulation. Obstruction of the stent due to tumor ingrowth occurred only in a noncovered stent (Fig. 1).
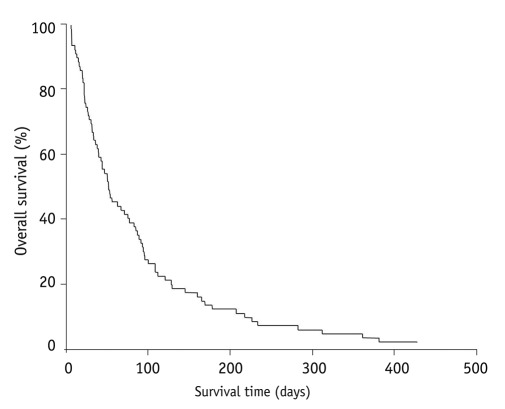
Eighty-one of 82 patients died during the follow-up period. The median survival time after stenting was 52 days (6-445) (Fig. 2). The median survival time of patients with noncovered SEMS placement and covered were 54 and 47 days, respectively. Also, there was no statistically significant difference between these two groups (Fig. 3). The obstruction of the SEMS due to tumor ingrowth was observed in 10 patients with a median time from SEMS placement to obstruction of 129 days (range, 35-216).
Malignant obstructions of the upper GI tract are frequent complications of advanced primary or metastatic tumors, generally as a terminal event in the malignancies, with poor life expectancy. In our series the most common location of malignant obstruction was the esophagus (34.1%). Esophageal cancer is the sixth leading cause of death from cancer worldwide (11, 12). More than 50% of patients with esophageal cancer are inoperable at the time of diagnosis, either because of advanced disease or due to the presence of comorbid conditions. SEMS placement has been widely accepted to be an effective option for palliation of the symptoms caused by malignant esophageal strictures. Several studies have reported excellent results in relief of dysphagia using SEMS (13-15). SEMS insertion is an effective way of relieving dysphagia in patients with inoperable carcinoma esophagus but it is not without complications. Perforation is one of most serious complication in patients with malignant dysphagia, and mortality rate is high. We also experienced perforation in one patient with esophageal obstruction after chemoradiation therapy and it was fatal.
The most common malignancy in our series was gastric cancer, and the number of patients with GOO was 43 (52.4%). Malignant GOO remains a challenging clinical condition. The main goal of the treatment is amelioration of the obstructive symptoms and the resumption of oral intake. Presently, standard treatment is palliative surgery, eg, gastrojejunostomy. Given the apparent preterminal nature of this event and short life expectancy, less invasive treatment, such as enteral stent placement, would appear favorable. In fact, endoscopic enteral stent placement has been stated to be a feasible, safe, effective alternative. Two recent meta-analyses comparing enteral stent placement with gastrojejunostomy have also confirmed the high technical and clinical success rates of stent placement and indicated favorable short-term outcomes with stenting (16, 17). In 2002, Adler and Baron (10) introduced the GOOSS scale for grading the clinical degree of gastric outlet obstruction both before and after treatment, and that scale is being increasingly adopted. A number of studies have demonstrated improved GOOSS results after stent placement (18-24). In our review, the GOOSS score showed a statistically significant improvement before (mean, 0.56) and after (mean, 1.92) (p < 0.001) SEMS placement. Although tumor ingrowth is the main cause of recurrent obstruction with noncovered SEMS, stent migration occurs more often with covered stents than with noncovered ones because the smooth surface of the covering reduces friction between the stent and the bowel wall (20, 25). We used noncovered stents for the palliative endoscopic treatment of malignant GOO to prevent migration. In our review, the median time from SEMS placement to obstruction due to tumor ingrowth (129 days) was longer than median survival time (52 days). Therefore, noncovered SEMS should be placed in patients with a short life expectancy to prevent migration. Here we reviewed SEMS placement in 82 patients with upper GI tract obstruction. Results were comparable with those of previous studies in terms of technical success (96.3%), clinical success (97.5%), rate of sever complication (perforation) (1.2%), and rate of stent obstruction due to tumor ingrowth (12.2%). However median survival time was shorter than that of previous studies (10, 20, 21). As a reason for that, 50% of all patients were aged above 70 years old in our review.
In summary, our review of SEMS placement for palliation of malignant upper GI obstruction showed comparable outcomes and frequency of complications, such as migration, obstruction, and perforation to previous studies. Our data also confirm that SEMS placement for malignant upper GI obstruction is an effective and safe procedure, with good clinical outcome.
References
1. Sabharwal T, Irani FG, Adam A. Cardiovascular and Interventional Radiological Society of Europe. Quality assurance guidelines for placement of gastroduodenal stents. Cardiovasc Intervent Radiol. 2007; 30:1–5. PMID: 17103108.

2. Mehta S, Hindmarsh A, Cheong E, Cockburn J, Saada J, Tighe R, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006; 20:239–242. PMID: 16362479.

3. Yim HB, Jacobson BC, Saltzman JR, Johannes RS, Bounds BC, Lee JH, et al. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc. 2001; 53:329–332. PMID: 11231392.

4. Wong YT, Brams DM, Munson L, Sanders L, Heiss F, Chase M, et al. Gastric outlet obstruction secondary to pancreatic cancer: surgical vs endoscopic palliation. Surg Endosc. 2002; 16:310–312. PMID: 11967685.
5. Maetani I, Tada T, Ukita T, Inoue H, Sakai Y, Nagao J. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy. 2004; 36:73–78. PMID: 14722859.

6. Mittal A, Windsor J, Woodfield J, Casey P, Lane M. Matched study of three methods for palliation of malignant pyloroduodenal obstruction. Br J Surg. 2004; 91:205–209. PMID: 14760669.

7. Maetani I, Akatsuka S, Ikeda M, Tada T, Ukita T, Nakamura Y, et al. Self-expandable metallic stent placement for palliation in gastric outlet obstructions caused by gastric cancer: a comparison with surgical gastrojejunostomy. J Gastroenterol. 2005; 40:932–937. PMID: 16261429.

8. Del Piano M, Ballarè M, Montino F, Todesco A, Orsello M, Magnani C, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc. 2005; 61:421–426. PMID: 15758914.

9. Fiori E, Lamazza A, Volpino P, Burza A, Paparelli C, Cavallaro G, et al. Palliative management of malignant antro-pyloric strictures. Gastroenterostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004; 24:269–271. PMID: 15015607.
10. Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002; 97:72–78. PMID: 11808972.

12. Pisani P, Parkin DM, Bray F, Ferlay J. Erratum: Estimates of the worldwide mortality from 25 cancers in 1990. Int. J. Cancer, 83, 18-29 (1999). Int J Cancer. 1999; 83:870–873. PMID: 10602059.
13. Sabharwal T, Hamady MS, Chui S, Atkinson S, Mason R, Adam A. A randomised prospective comparison of the Flamingo Wallstent and Ultraflex stent for palliation of dysphagia associated with lower third oesophageal carcinoma. Gut. 2003; 52:922–926. PMID: 12801944.

14. Laasch HU, Marriott A, Wilbraham L, Tunnah S, England RE, Martin DF. Effectiveness of open versus antireflux stents for palliation of distal esophageal carcinoma and prevention of symptomatic gastroesophageal reflux. Radiology. 2002; 225:359–365. PMID: 12409567.

15. Vakil N, Morris AI, Marcon N, Segalin A, Peracchia A, Bethge N, et al. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. Am J Gastroenterol. 2001; 96:1791–1796. PMID: 11419831.

16. Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007; 42:283–290. PMID: 17464457.

17. Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007; 7:18. PMID: 17559659.

18. Jeurnink SM, Steyerberg EW, Hof G, van Eijck CH, Kuipers EJ, Siersema PD. Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: a comparison in 95 patients. J Surg Oncol. 2007; 96:389–396. PMID: 17474082.

19. Schiefke I, Zabel-Langhennig A, Wiedmann M, Huster D, Witzigmann H, Mössner J, et al. Self-expandable metallic stents for malignant duodenal obstruction caused by biliary tract cancer. Gastrointest Endosc. 2003; 58:213–219. PMID: 12872088.

20. Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004; 36:543–550. PMID: 15202052.

21. Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc. 2004; 60:1010–1017. PMID: 15605026.

22. Lee SM, Kang DH, Kim GH, Park WI, Kim HW, Park JH. Self-expanding metallic stents for gastric outlet obstruction resulting from stomach cancer: a preliminary study with a newly designed double-layered pyloric stent. Gastrointest Endosc. 2007; 66:1206–1210. PMID: 18028923.

23. Lowe AS, Beckett CG, Jowett S, May J, Stephenson S, Scally A, et al. Self-expandable metal stent placement for the palliation of malignant gastroduodenal obstruction: experience in a large, single, UK centre. Clin Radiol. 2007; 62:738–744. PMID: 17604761.

24. Maetani I, Isayama H, Mizumoto Y. Palliation in patients with malignant gastric outlet obstruction with a newly designed enteral stent: a multicenter study. Gastrointest Endosc. 2007; 66:355–360. PMID: 17643712.

25. Kim GH, Kang DH, Lee DH, Heo J, Song GA, Cho M, et al. Which types of stent, uncovered or covered, should be used in gastric outlet obstructions? Scand J Gastroenterol. 2004; 39:1010–1014. PMID: 15513343.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download