Abstract
Objective
To demonstrate a comprehensive review of published articles regarding endoscopic ultrasound (EUS)-guided biliary drainage.
Materials and Methods
Review of studies regarding EUS-guided biliary drainage including case reports, case series and previous reviews.
Results
EUS-guided hepaticogastrostomy, coledochoduodenostomy and choledoantrostomy are advanced biliary and pancreatic endoscopy procedures, and together make up the echo-guided biliary drainage. Hepaticogastrostomy is indicated in cases of hilar obstruction, while the procedure of choice is the coledochoduodenostomy or choledochoantrostomy in distal lesions. Both procedures must be performed only after unsuccessful ERCPs. The indication of these procedures must be made under a multidisciplinary view while sharing information with the patient or legal guardian.
Endoscopic biliary stenting at ERCP is a well-established therapy for both benign and malignant biliary obstructions (1-3). Biliary cannulation fails in 10-15% of cases due to several reasons including ampullary pathology (tumor, stenosis, stones), periampullary diverticulum, anatomic variations or in patients where the papilla cannot be reached by the duodenoscope as in gastric outlet obstruction and surgically altered anatomy (gastric bypass, Roux-en-Y reconstruction). To overcome ERCP failures and improve outcomes over those afforded by more invasive alternatives - percutaneous transhepatic biliary drainage (PTBD) and surgery, as well as endoscopic ultrasound (EUS)-guided ductal access techniques paired with standard ERCP drainage techniques, have been developed in recent years. This hybrid procedure is given a variety of names, but the more encompassing one is endosonographic cholangiopancreatography (ESCP)(4). Based on the combination of the three possible access routes (intrahepatic bile duct, extrahepatic bile duct, and pancreatic duct) with the three possible drainage routes (transmural, transpapillary antegrade, and transpapillary retrograde), ESCP admits several variant approaches for both pancreatic and biliary drainage; the last also referred to collectively as EUS-guided biliary drainage (EUSBD).
The rationale for all variant EUSBD approaches, as a second-line option in select difficult cases where ERCP is not feasible, is threshold. EUSBD may be potentially more convenient because it is performed in the same session, more physiological given that it allows immediate internal biliary drainage, and less invasive given that it affords a more accurate control as well as more access sites to the bile duct than the classical alternatives of PTBD or surgery.
EUS-BD is an invasive and complex procedure. Knowledge about the full array of needle devices, guidewires, dilators and stents, as well as the subtle variations in scope position (gastric or duodenal), scope orientation (upward and downward), and stent anchoring techniques, is highly recommended for increasing procedure success rates and minimize complications.
EUS-guided hepatico-gastrostomy (HG) was first reported in 2003 by Burmester et al. with the performance of EUS-HG in a Billroth II patient with unresectable pancreatic cancer and failed ERCP due to tumor infiltration of the papilla.
EUS-HG is closely related to EUS-guided drainage of pancreatic pseudocysts (5). The procedure consists of identification of the bile duct by EUS, followed by a puncture with a needle. The puncture tract is then dilated using cautery, mechanical devices, or both, and a stent is placed across the puncture tract to drain the duct into the GI tract lumen.
The procedural steps of EUS-HG are as follows: Using an interventional echoendoscope, the dilated left hepatic duct (usually segment III) is well visualized. EUS-HG is then performed under combined fluoroscopic and ultrasound guidance, with the tip of the echoendoscope positioned such that the ultrasound transducer is either in the middle part of the small curvature of the stomach or slightly upwards, closer to the cardia. A 19G FNA needle is inserted transgastrically into a peripheral branch of the left hepatic duct, and contrast medium is injected. Before injecting the contrast fluid, bile can be aspirated through the needle in order to confirm the intraductal position of the needle tip. Opacification delineates fluoroscopically the dilated biliary tree above the point of obstruction. The needle is exchanged over a guidewire for a 6.0-Fr diathermic sheath, which is then used to enlarge the channel between the stomach (or jejunum in patients with total gastrectomy), and the left hepatic duct. The diathermic sheath is advanced across the intervening liver parenchyma by using a cutting current. After removing the guidewire, the diathermic sheath, which is an 8.5-Fr, 8-cm - long hepatico-gastric stent) or an 8 cm long covered self-expandable metal stent (SEMS), partially covered or fully covered, is placed transmurally. Fluoroscopy confirms adequate stent placement and function by showing contrast drainage through the stent into the stomach (Fig. 1).
Bile leakage into the peritoneum is the major risk factor for EUS-HG. Several strategies are used by different authors to minimize this risk. A 6 or 7-Fr naso-biliary drain with mild aspiration or gravity drainage can be left in place through the metal stent over 48 hours, even if this is somewhat inconvenient to the patient. More recently, this method has been developed into a more patient-friendly approach to minimize the risk of leakage, by combining an uncovered metal stent with a covered metal stent inside. The uncovered stent is initially deployed to provide anchorage and prevent migration. The covered stent is inserted coaxially and deployed within the first stent. Finally, in cases where the guidewire crosses the downstream stricture antegradely, hepaticogastrostomy can be combined with antegrade placement of an additional metal stent bridging in the distal stricture, which further decreases the pressure gradient across the transmural stent by providing additional downstream decompression of the bile duct (6). Alternative strategies used by other authors to prevent migration include the placement of fully covered SEMS with both ends flared (7), or forceful balloon expansion upon stent deployment (as opposed to gradual spontaneous self-expansion over several hours) to monitor foreshortening and the insertion of a double pig-tail stent through the expanded SEMS in order to provide additional anchorage (8).
The specific anatomic features of patients that may make EUS-HG preferable to other EUSBD are based on the intrahepatic access route and the transmural drainage route. Intrahepatic access is the only choice in patients with proximal (hilar) biliary obstruction, and is usually more convenient in patients with distal gastrectomy since imaging the common bile duct (CBD) under EUS is not always possible in postoperative altered anatomy setting (9). One advantage of transmural drainage after intrahepatic bile duct access over transpapillary drainage is that the challenging step of antegrade guidewire passage (required for both rendezvous and antegrade stenting) is avoided. In addition to guidewire passage, rendezvous requires an accessible papilla, which is usually not the case in patients with a surgically altered anatomy or tight duodenal stenosis. Antegrade stent insertion does not require an accessible papilla, but involves dilation of the puncture tract, just as EUS-HG. In patients with postoperative anatomy, antegrade transpapillary stenting without combined hepaticogastrostomy is less convenient for stent revisions, since HG provides easy repeat access to the bile duct without the need for a repeat puncture. Stent revisions are not uncommonly required during follow-up. The advantages of EUS-HG over rendezvous or antegrade stent insertion are particularly relevant in patients with prior duodenal or biliary SEMS who experience recurrent biliary obstruction (10).
However, these variant EUSBD approaches should be viewed as complementary rather than mutually exclusive. For example, as mentioned when discussing strategies to minimize the risk of bile leakage in EUS-HG, antegrade transpapillary stents can be combined with transmural stenting (6). Püspök et al. (11) performed an antegrade transpapillary SEMS insertion in a patient with recurrent gastric cancer after a Roux-en-Y gastrectomy. The authors then left a transmural plastic stent across the puncture tract to both minimize the risk of leakage and to preserve access. Dual drainage (antegrade and transmural) has also been used serially. Fujita et al. (12) performed transesophageal EUSBD by inserting a 7-Fr plastic stent into a peripheral left bile duct branch in a patient with advanced gastric cancer. Ten days later, the plastic stent was cannulated with a guidewire and removed with a snare (13). Then, using flexible devices through the mature fistula, the guidewire was manipulated under fluoroscopy across the malignant distal bile duct stricture, and a SEMS passed antegradely over the wire was subsequently deployed across the stricture above the papilla.
Patients with distal bile duct obstruction without prior gastrectomy who have both intra and extrahepatic bile duct dilations (and no gross ascites), are the only cases in which an intrahepatic or extrahepatic access site for EUSBD might be preferable if the selection criteria for EUSBD versus PTBD are broad, [i.e., EUSBD is favoured as the initial second-line approach after failed ERCP, and this type of patient may represent just 20% of the candidate population (14)]. Operator preference plays a part in this small patient subset. CBD offers a more obvious target for an EUS puncture, the echoendoscope is in a more anchored position, and access to the CBD probably makes rendezvous easier than for intrahepatic access. On the other hand, intrahepatic EUSBD is performed with an echoendoscope in a more straight position, which favors the transmission of the pushing force during stent insertion. It is also probably easier to penetrate a small intrahepatic bile duct surrounded by liver parenchyma than the fibrotic, hard wall of the CBD.
To date, transmural intrahepatic EUSBD has been reported in 51 patients, EUS-HG in 42, and other closely related variant approaches through a transjejunal or a transesophageal route in 9. In five patients with total gastrectomy, the left bile duct was similarly accessed under EUS from below the cardia and transmural stents were placed across the jejunal wall. In the remaining four patients, a cephalad peripheral left bile duct branch was selected for puncture, so that eventually the stent pierced the wall of the intra-abdominal esophagus slightly above the cardia. Approximately half of these patients come from three small series, which specifically deal with transmural intrahepatic EUSBD (6, 10, 15), whereas the other half comes from either mixed series in which EUS-HG is reported along extrahepatic EUSBD (7, 11, 16-19) or individual case reports (12, 20-24) (Table 1).
EUS-HG (or its variants) was technically successful in 49 out of these 51 patients, with clinical resolution of biliary obstruction in 46 cases. Therefore EUS-HG had a 94% per-protocol success rate and a 90.2% success rate on an intention-to-treat basis. These success rates are very high, considering the difficult patient population in which EUS-HG was attempted. However, it is important to remember that success was achieved at the expense of an overall 20% complication rate, twice as high as that of ERCP. Most complications were accounted for by inadequate biliary drainage, resulting in either peritoneal bile leakage or cholangitis (Table 1). Plastic stents caused cholangitis due to early migration (19) or early clogging (6). Foreshortening of transmural SEMS led to bile perotinitis or biloma, requiring percutaneous drainage and repeat EUSBD (6), and caused the only reported death to date (24). Half of the complications were nonetheless mild, manifesting as transient abdominal pain with or without pneumoperitoneum that settled on conservative measures.
There is great consistency across all reports on EUS-HG regarding technical details except for the use of cautery, be it kneedle-knives or fistulotomes and the choice of the stent. Overall, any diathermy use was reported in just 39.5% of cases, with some authors using it routinely (6), and others resorting to it selectively (7) (only after failure to advance a mechanical dilator over the guidewire), or do not use it at all (15).
As detailed in Table 1, 7 to 8.5-Fr plastic stents were placed in 46% of cases, whereas uncovered, partially covered or fully covered SEMS were placed initially in 54% of cases. It is difficult to draw significant conclusions from published reports, since no formal comparisons have been made between the two types of stents. SEMS are appealing for three reasons. First, upon full expansion, SEMS effectively seals the puncture/dilation tract, which would in theory prevent leakage more effectively. Secondly, their larger diameter provides better long-term patency, which would decrease the need for stent revisions. Finally, if dysfunction by ingrowth or clogging occurs, management is somewhat less challenging than with plastic stents, since a new stent (plastic or SEMS) can easily be inserted through the occluded SEMS in place. In contrast, exchanging a clogged plastic transmural stent usually requires over-the-wire replacement, because free-hand removal involves the risk of track disruption with subsequent guidewire passage into the peritoneum, hence requiring repeat EUSBD (or PTBD) to re-establish drainage (13). These presumed advantages of SEMS must be balanced against the fact that transmural SEMS insertion and deployment are somewhat more demanding than they are for ERCP. In particular, the serious risk of foreshortening and bile peritonitis should be prevented with careful attention to detail (24).
EUS-guided extrahepatic bile duct drainage is performed in a procedure where the CBD is punctured from a transduodenal or transgastric approach (usually from the distal antrum). The CBD is more easily imaged under EUS than the intrahepatic bile ducts, providing a faster, cleaner access without repeated puncture attempts, thereby minimizing risks. The retroperitoneal location of the CBD makes it also an attractive access site for patients with ascites, in whom fluid around the liver makes transhepatic access (whether percutaneous or transgastric under EUS) more difficult and hazardous.
Besides the advantages of extrahepatic access over intrahepatic access, the specific rationale for EUS-Choledochoduodenostomy (CDS) is also derived from the transmural drainage route, as opposed to transpapillary EUSBD (antegrade or rendezvous) (11, 25). The real choice between transmural and transpapillary drainage after extrahepatic bile duct access under EUS, therefore lies between EUS-CDS and rendezvous. Proponents of rendezvous argue that it may be less invasive than EUS-CDS, since transmural interventions are usually limited to puncture and guidewire passage. Hence, drainage is accomplished retrogradely via ERCP without the need for puncture tract dilation (26). However, EUSBD rendezvous carries a 20% failure rate; even in expert centers, because guidewire passage across the stricture and the papilla is often unsuccessful. The needle allows virtually no interplay with the guidewire, which cannot be manipulated across the stricture through a needle in the same way as it can be done at ERCP using flexible catheters. Therefore, advantages of EUS-CDS over transpapillary rendezvous are its higher success rate and relative simplicity, which appear to make it a more reproducible approach, despite being perhaps more invasive. Nonetheless, both EUSBD variant approaches can be considered complementary inasmuch as these procedures are used in a heterogeneous patient population. As we will discuss below, some indications are better suited for EUS-CDS, whereas in other cases EUSBD rendezvous is clearly advantageous. Similarly, even if rendezvous is the intended drainage technique, EUS-CDS can be used as a second line approach to salvage the significant proportion of failed rendezvous cases (17, 27). This open-ended approach to EUSBD (i.e., including both rendezvous and EUS-CDS), results in comparatively higher success rates than that of an EUSBD series, hence limiting their approach to just rendezvous (26). It is important to know that choledochoantrostomy, described by Artifon et al. (28), is a new technique that is useful for those patients with duodenal bulb infiltration, and may be a new and feasible tool as a variant of choledochoduodenostomy.
In common with other EUSBD techniques, EUS-CDS should only be considered in patients with confirmed (not just suspected) biliary obstruction after a failed ERCP and despite maximal attempts by experienced operators.
EUS-CDS has specific anatomic requirements differing from other EUSBD alternatives. The first anatomic requirement is distal biliary obstruction. In other words, EUS-CDS is not suitable for proximal (hilar) biliary obstruction where intrahepatic EUSBD approaches are clearly required. The second anatomic requirement is the ability to image the CBD under EUS. Since the CBD is typically imaged from the distal stomach or the duodenal bulb, this is difficult or impossible in patients with prior gastrectomy and gastrojejunostomy (e,g, Roux-en-Y) (9).
Finally, as with most other EUSBD approaches, EUS-CDS is predominantly used in patients with malignant biliary obstruction. Where alternative approaches such as rendezvous may rightly be considered after failed cannulation in patients with documented benign causes of biliary obstruction (eg, CBD stones or papillary stenosis), EUS-CDS is less adequate in these distinct settings, where biliary drainage is usually accomplished by means of a sphincterotomy (with or without stone removal) as opposed to stenting.
Puncture of the CBD from the duodenum (EUS-CDS) is the most common approach for biliary drainage. A similar approach from the stomach (EUS-choledochogastrostomy or EUS-choledochoantrostomy) may also be used in selected instances depending on the patient's anatomy. The orientation of the needle should be checked with fluoroscopy before the puncture is actually carried out. EUS-guided puncture can be made with different sized needles, mostly 19G and 22G, which are inserted transduodenally into the bile duct under EUS visualization. To confirm needle ductal access, the stylet is removed and bile is aspirated. If there is a bile return, contrast medium is injected into the bile duct for cholangiography, and then, a 450 cm long guidewire is inserted through the outer sheath and its position is confirmed fluoroscopically (Fig. 2).
After guidewire access into the bile duct, some dilation of the puncture track is usually necessary, using either a dilating biliary catheter, a papillary balloon dilator or two types of needles commonly used at ERCP such as needle-knife or cystotome. This is aimed at dilating the choledochoduodenal fistula to facilitate stent insertion. Finally, a 5-Fr to 10-Fr plastic stent (straight or pigtail) or a fully covered SEMS is inserted through the choledochoduodenostomy site into the CBD. Care should be taken to monitor, by fluoroscopy, the intraductal placement of the proximal end of the stent and to monitor by endoscopy the intraduodenal (or intragastric) position of the distal (closer to the scope) end of the stent. This latter aspect is of particular relevance when using SEMS. To prevent SEMS dislodgment, an adequate length of SEMS (15-20 mm) should be left inside the GI lumen. Additional anchorage techniques to prevent dislodgment are forceful balloon dilation of the SEMS up to 8-10 mm after initial deployment, or the use of a coaxial double pig-tail through the SEMS, as reported for pseudocyst drainage using transmural SEMS (29).
Operator confidence with specific devices also plays a role in the success of the method. Some authors feel that access without cautery is less prone to complications and for performing mechanical dilation, which requires a stiffer 0.035-inch guidewire for support, subsequently use of a 19-gauge EUS-FNA needle. These authors use access with cautery, only selectively after failed mechanical dilation (7, 8).
Other authors find the stiffer 19-gauge EUS-FNA needles to be cumbersome to use in the relatively long position of the echoendoscope in the duodenum, and resort to either initial direct needle-knife access under EUS (30), or needle-knife access under a thinner 0.018-inch guidewire passed into the CBD after puncture with a 22-gauge EUS-FNA needle (31).
EUS-guided choledochoduodenostomy was first reported by Giovannini et al. (32). As detailed earlier, the puncture needles available consist of conducting and nonconducting needles. About half of each kind of needle has been used in published reports. This is in contrary to what is reported for intrahepatic EUSBD, where nonconducting needle access is clearly preferred.
In most reported cases, a plastic stent has been placed. However, the use of SEMS has increasingly been reported (7). The success rate for the 61 cases reported to date is as high as 95%, with excellent results in all successfully drained patients (100% per-protocol clinical response rate). There were some cases where stent insertion was too difficult and a nasobiliary drainage tube was placed instead (19, 33). Another interesting variation on EUS-CDS is illustrated by a few cases where the extrahepatic bile duct was punctured from the stomach rather than the standard transduodenal approach (7, 34). Although only 6 cases were reported, all were successful.
Complications can be divided into procedure-related complications and stent-related complications. Definitions of procedural complications are not well standardized. Most are related to bile or just air leakeage into the retroperitoneum by transduodenal access or the peritoneum by transgastric access to the CBD, with or without the added infection. The severity ranges from a selflimiting condition that resolves within 48-72 hours with conservative measures, to full-blown peritonitis requiring emergency surgery; however, most reported complications are mild and the need for emergency surgery is exceedingly rare. Other interventional measures that may be required in the event of complications, such as percutaneous drainage, are however not all that uncommon.
Peri-procedural leakage of bile into the abdominal cavity is most likely due to poor drainage. Poor drainage can be caused by factors such as too large a fistula, early stent clogging, and inappropriate positioning of the stent including foreshortening of SEMS.
Late stent-related complications, since a mature fistula is formed, are similar to those seen with transpapillary stents placed at ERCP, namely, migration and stent occlusion. Stent migration or occlusion is managed as well the same way as in stents placed at ERCP, by inserting a new stent. The technique for repeat stent placement differs from what is commonly done at ERCP. If a clogged plastic stent is in place across the fistula, a guidewire is advanced through the stent and the stent is grasped with a snare passed over-the-wire and removed over it. This somewhat more complex maneuver is aimed at keeping guidewire access to the duct after stent removal. After plastic stent removal, a SEMS may be placed using a duodenoscope. If clogging of a SEMS occurs, the debris occluding its lumen may be cleaned up. However, just cleaning is probably not long-lasting in this setting. A new coaxial stent needs to be placed inside the clogged one, either a plastic stent, or a SEMS, the so-called stent-in-stent approach.
Distal stent migration into the GI tract lumen with a mature fistula only involves repeat biliary drainage, since migrated stents usually pass out spontaneously. Repeat biliary drainage may be attempted in several ways, the simplest being placing a new stent through the same fistula, if it is still visible. If the fistula cannot be identified endoscopically, either repeat EUS-CDS through a new puncture site or PTBD are required. If proximal stent migration to the retroperitoneum or the peritoneum occurs, recovery of the stent as well as emergency surgery should be considered. This serious complication, however, has not yet been reported for EUS-CDS. Finally, even if the less serious distal migration occurs but the fistula is still immature (a fibrous track not yet formed), this may cause bile leakage into the abdomen. In the event of stent migration and leakage with an immature fistula, repeat EUS-guided biliary drainage (perhaps using a SEMS), or PTBD need to be considered. Surgery should also be considered depending on the patient's condition.
Interventional EUS techniques for biliary drainage complement ERCP to achieve biliary access and drainage, but are invasive procedures that require careful patient selection and experienced operators backed by a multidisciplinary team.
Further technical improvements are likely to reduce the number of adverse events and will probably contribute to the more widespread adoption of these procedures as a second-line approach to biliary drainage after failed ERCP.
The readers also could appreciate a new EUS technology in which a forward view of ultrasound image is possible, and may facilitate the acquirement of an appropriate window to the therapeutic EUS (35). A short double face metal stent may be used to create a fistulisation between the dilated biliary tree and duodenum; this kind of stent is named an AXIOS® Stent (X Lumena), and is also useful for treatment of pancreatic pseudocysts.
Although multicenter trials aimed at standardizing the technique for performing EUS-guided biliary drainage would be desirable, the relatively few patient candidates for it and the wide spectrum of technical variations reported to date make this endeavor difficult to accomplish in the near future. Detailed prospective studies with homogeneous inclusion criteria and careful follow-up and dedicated hands-on training models will probably be more effective in advancing this burgeoning field of interventional endoscopy.
Figures and Tables
Fig. 1
Endoscopic ultrasound (EUS)-guided hepatico gastrostomy is demonstrated using step-by-step technique in which we can see EUS images with dilated IHD, puncture, cholangiography, guidewire placement, fistulization using needle-knife catheter, and deployment of partially covered self-expandable metal stent.
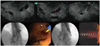
Fig. 2
Endoscopic ultrasound (EUS)-guided choledochoduodenostomy. It is demonstrated step-by-step technique in which we can see EUS images with dilated common bile duct, puncture, cholangiography, guidewire placement, fistulization using needle-knife catheter, deployment of partially covered self-expandable metal stent.
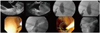
Table 1
Summary of Published Literature on EUS-HG and Related Transmural Intrahepatic EUSBD Techniques

Note.- Case reports from Giovannini et al. (20) and Fujita et al. (12) are not tallied because they are already included in case series by Bories et al. (6) and Horaguchi et al. (19), respectively. EUSBD = endoscopic ultrasound-guided biliary drainage, HG = hepatico-gastrostomy, Pneumo = pneumoperitoneum, SEMS = self-expandable metal stent
References
1. Fogel EL, Sherman S, Devereaux BM, Lehman GA. Therapeutic biliary endoscopy. Endoscopy. 2001. 33:31–38.
2. Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994. 344:1655–1660.
3. Lai EC, Mok FP, Tan ES, Lo CM, Fan ST, You KT, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992. 326:1582–1586.
4. Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK. Endosonography-guided cholangiopancreatography. Gastrointest Endosc. 1996. 43:102–106.
5. Giovannini M. EUS-guided pancreatic pseudocyst drainage. Tech Gastrointest Endosc. 2007. 9:32–38.
6. Bories E, Pesenti C, Caillol F, Lopes C, Giovannini M. Transgastric endoscopic ultrasonography-guided biliary drainage: results of a pilot study. Endoscopy. 2007. 39:287–291.
7. Park do H, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, et al. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009. 104:2168–2174.
8. Perez-Miranda M, de la Serna C, Diez-Redondo P, Vila JJ. Endosonography-guided cholangiopancreatography as a salvage drainage procedure for obstructed biliary and pancreatic ducts. World J Gastrointest Endosc. 2010. 2:212–222.
9. Wilson JA, Hoffman B, Hawes RH, Romagnuolo J. EUS in patients with surgically altered upper GI anatomy. Gastrointest Endosc. 2010. 72:947–953.
10. Park do H, Song TJ, Eum J, Moon SH, Lee SS, Seo DW, et al. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos). Gastrointest Endosc. 2010. 71:413–419.
11. Püspök A, Lomoschitz F, Dejaco C, Hejna M, Sautner T, Gangl A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: a case series. Am J Gastroenterol. 2005. 100:1743–1747.
12. Fujita N, Noda Y, Kobayashi G, Ito K, Obana T, Horaguchi J, et al. Temporary endosonography-guided biliary drainage for transgastrointestinal deployment of a self-expandable metallic stent. J Gastroenterol. 2008. 43:637–640.
13. Fujita N, Sugawara T, Noda Y, Kobayashi G, Ito K, Obana T, et al. Snare-over-the-wire technique for safe exchange of a stent following endosonography-guided biliary drainage. Dig Endosc. 2009. 21:48–52.
14. Perez-Miranda M, De la Serna C, Diez-Redondo MP, Gomez de la Cuesta S, Gil-Simon P, Alcaide N, et al. Endosonography-guided cholangiopancreatography (ESCP) as the primary approach for ductal drainage after failed ERCP. Gastrointest Endosc. 2010. 71:AB136.
15. Will U, Thieme A, Fueldner F, Gerlach R, Wanzar I, Meyer F. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007. 39:292–295.
16. Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003. 57:246–251.
17. Maranki J, Hernandez AJ, Arslan B, Jaffan AA, Angle JF, Shami VM, et al. Interventional endoscopic ultrasound-guided cholangiography: long-term experience of an emerging alternative to percutaneous transhepatic cholangiography. Endoscopy. 2009. 41:532–538.
18. Eum J, Park do H, Ryu CH, Kim HJ, Lee SS, Seo DW, et al. EUS-guided biliary drainage with a fully covered metal stent as a novel route for natural orifice transluminal endoscopic biliary interventions: a pilot study (with videos). Gastrointest Endosc. 2010. 72:1279–1284.
19. Horaguchi J, Fujita N, Noda Y, Kobayashi G, Ito K, Obana T, et al. Endosonography-guided biliary drainage in cases with difficult transpapillary endoscopic biliary drainage. Dig Endosc. 2009. 21:239–244.
20. Giovannini M, Dotti M, Bories E, Moutardier V, Pesenti C, Danisi C, et al. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy. 2003. 35:1076–1078.
21. Artifon EL, Chaves DM, Ishioka S, Souza TF, Matuguma SE, Sakai P. Echoguided hepatico-gastrostomy: a case report. Clinics (Sao Paulo). 2007. 62:799–802.
22. Chopin-Laly X, Ponchon T, Guibal A, Adham M. Endoscopic biliogastric stenting: a salvage procedure. Surgery. 2009. 145:123.
23. Iglesias-García J, Lariño-Noia J, Seijo-Ríos S, Domínguez-Muñoz JE. Endoscopic ultrasound for cholangiocarcinoma reevaluation after Wallstent placement. Rev Esp Enferm Dig. 2008. 100:236–237.
24. Martins FP, Rossini LG, Ferrari AP. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: fatal complication. Endoscopy. 2010. 42:Suppl 2. E126–E127.
25. Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010. 42:232–236.
26. Kim YS, Gupta K, Mallery S, Li R, Kinney T, Freeman ML. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy. 2010. 42:496–502.
27. Brauer BC, Chen YK, Fukami N, Shah RJ. Single-operator EUS-guided cholangiopancreatography for difficult pancreaticobiliary access (with video). Gastrointest Endosc. 2009. 70:471–479.
28. Artifon EL, Okawa L, Takada J, Gupta K, Moura EG, Sakai P. EUS-guided choledochoantrostomy: an alternative for biliary drainage in unresectable pancreatic cancer with duodenal invasion. Gastrointest Endosc. 2011. 73:1317–1320.
29. Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, Kahaleh M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest Endosc. 2008. 68:1199–1203.
30. Yamao K, Sawaki A, Takahashi K, Imaoka H, Ashida R, Mizuno N. EUS-guided choledochoduodenostomy for palliative biliary drainage in case of papillary obstruction: report of 2 cases. Gastrointest Endosc. 2006. 64:663–667.
31. Tarantino I, Barresi L, Repici A, Traina M. EUS-guided biliary drainage: a case series. Endoscopy. 2008. 40:336–339.
32. Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001. 33:898–900.
33. Itoi T, Itokawa F, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, et al. Endoscopic ultrasound-guided choledochoduodenostomy in patients with failed endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2008. 14:6078–6082.
34. Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006. 64:52–59.
35. Kida M, Araki M, Miyazawa S, Ikeda H, Kikuchi H, Watanabe M, et al. Fine needle aspiration using forward-viewing endoscopic ultrasonography. Endoscopy. 2011. 43:796–801.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download