Abstract
Endoscopic therapy by balloon dilation and placement of multiple large-bore plastic stents is the treatment of choice for benign biliary stricture. This approach is effective but it typically requires multiple endoscopic sessions given the short duration of stent patency. The endoscopic approach for treatment of bile leak involves the placement of a stent with or without biliary sphincterotomy. The self-expandable metal stent (SEMS) has traditionally been used for palliation of malignant biliary strictures given the long duration of stent patency owing to their larger stent diameter. Recently, SEMS has been used in a variety of benign biliary strictures and leaks, especially with the design of the covered self-expandable metal stent (CSEMS), which permits endoscopic-mediated stent removal. The use of CSEMS in benign biliary stricture could potentially result in a decrease in endoscopic sessions and it is technically easier when compared to placement of multiple plastic stents. However, complications such as cholecystitis due to blockage of cystic duct, stent migration, infection and pancreatitis have been reported. The potential subsegmental occlusion of contralateral intrahepatic ducts also limits the use of CSEMS in hilar stricture. Certain techniques and improvement of stent design may overcome these challenges in the future. Thus, CSEMS may be appropriate in only highly selected conditions, such as refractory benign biliary stricture, despite multiple plastic stent placement or difficult to treat bile duct stricture from chronic pancreatitis, and should not be used routinely. This review focuses on the use of fully covered self-expandable metal stent for benign biliary strictures and bile leaks.
Benign biliary strictures (BBS) can be caused by postoperative injury, anastomotic injury following orthotopic liver transplantation (OLT), chronic pancreatitis, primary sclerosing cholangitis, and other inflammatory conditions (1-10) (Fig. 1). Postoperative strictures including cholecystectomy, common bile duct exploration, and anastomotic stricture following OLT are the most frequent (11). The clinical presentation of BBS can be broad including subclinical, abnormal liver function tests, abnormal imaging as characterized by bile duct dilatation, and complete biliary obstruction manifesting as abdominal pain, jaundice, and cholangitis. Patients suffering from postoperative stricture may present within a few days after surgery or have a delayed presentation if the stricture is minor. BBS is usually diagnosed by cross-sectional imaging such as computed tomography (CT), magnetic resonance cholangiopancreatography and exclusion of malignant condition. Bile leaks generally occur after hepato-biliary surgery with postcholecystectomy being the most common cause (12). Leakage of cystic duct and duct of Luschka is generally the cause of biliary leak after cholecystectomy. Endoscopic placement of biliary plastic stents has long been widely accepted as the treatment of choice for benign biliary stricture and leaks. Recent data, however, have shown that covered SEMS (CSEMS) may offer benefits and can be used for these conditions (6, 7, 13-16).
Endoscopic therapy by placing multiple plastic stents with or without balloon dilation is very effective in postoperative strictures, including post OLT and cholecystectomy, while the treatment outcomes are less favorable in chronic pancreatitis. Among the strictures related to post OLT, anastomotic strictures (AS) have a better response when compared to nonanastomotic strictures caused by ischemic injury (17). Location of strictures is also important in predicting treatment success. There are two major systems used for assessment of biliary stricture: the Bismuth system, which is based on the location of stricture, and the Strasberg classification, which is based on location and biliary leak (18, 19). Distally located BBS (below hepatic hilum) have a superior success rate than proximal BBS (stricture involving hepatic hilum) after endoscopic therapy (20).
Treatment options for BBS include surgery, percutaneous approach, and endoscopic therapy. The treatment goals should be to improve and maintain bile duct patency as well as preventing recurrent stricture by performing the least invasive procedure. Thus, the endoscopic approach has been accepted as the treatment of choice given its efficacy and less invasive nature, compared to surgery. Insertion of one or more plastic stents with or without dilation has been used as the standard treatment because of ease of removal and cost. To maximize the treatment outcomes, the treatment strategy involves placement of additional large-bore plastic biliary stents (10 French) over time and the stents can be exchanged periodically (every 3-6 months) for at least 1 year (1). Practically, patients would undergo at least three endoscopic sessions within a year to complete the treatment protocol, which is the major disadvantage of this approach. In addition, suboptimal stricture resolution and the development of cholangitis due to stent occlusion are the important limitations of this approach. Thus, the use of SEMS, with its' immediate radial expansion capability, has been proposed to lessen the number of endoscopic sessions. Also, the larger diameter of SEMS may potentially result in less stent occlusion and better stricture resolution. However, bare SEMS can create tissue hyperplasia and embedment, which would preclude stent removal, and so is not a recommended treatment for the benign condition. With the evolution of stent design, partially covered and fully covered SEMS have been developed. These stents allow temporary placement of SEMS and can therefore be used in BBS.
Studies have been conducted to assess stent patency, efficacy, removability, and complications of partially covered self-expandable metal stent (PCSEMS) in biliary strictures. A study of 79 patients who underwent placement of PCSEMS (Wallstent; Boston Scientific, Natick, MA, USA) for a variety of BBS including chronic pancreatitis, biliary stone disease, post-OLT, and post-surgery showed that overall success rate was about 90% after leaving the stents in place for 4 months. Stricture resolution rate was noted to be lowest in chronic pancreatitis. The authors were able to removal all stents after biliary decompression, as confirmed by clinical and laboratory investigation. Median follow-up time after stent removal was 12 months. Stent migration occurred in 14% of cases, and tissue hyperplasia at the proximal uncovered portion of the stent resulting in stricture was also noted in some patients (13). The usefulness of PCSEMS in management of post-OLT related stricture after failure of conventional endoscopic therapy has also been demonstrated. Vandenbroucke et al. (21) placed Wallstents in patients with biliary stricture due to post-OLT who failed adequate balloon dilation. Twelve-, 18-, and 24-month primary patency rate was 64%, 51%, and 26%, respectively. Two patients underwent retransplantation for diffuse ischemic cholangitis or chronic rejection. Tee et al. (22) reported two cases of AS refractory to the conventional endoscopic and/or surgical approach treated with insertion of a removable fully covered SEMS (Niti-S biliary stent; Taewoong Medical, Seoul, Korea). After successful deployment, the stent remained in situ for 42 and 70 days, and no stent migration was observed. During an 18-month follow-up, liver function tests remained stable without further episode of jaundice and cholangitis, and no other procedures were required. Furthermore, in a recent prospective uncontrolled multicenter study of 22 patients (14) to evaluate the placement and removal of PCSEMS in the setting of AS post-liver transplantation, stents were placed for 2 months and all were removed except two due to distal migration. Stent insertion related complications included mild pancreatitis, transient abdominal pain, and cholangitis. The authors encountered minor complications associated with stent removal including self-contained hemorrhage and fever. A sustained stricture resolution rate of 52.6% was observed based on per protocol analysis. The stricture persisted at the end of treatment in three patients (13.6%); all were related to premature stent migration or dislocation. Recurrence of stricture after the initial success occurred in 47.4% of cases. The efficacy of PCSEMS in terms of stricture resolution for treatment of common bile duct stricture due to chronic pancreatitis is more favorable at a short-term follow-up when compared to a plastic stent (6, 7). Despite an impressive initial response and a reasonably good stent patency at 12-20 months, the overall success rate of PCSEMS in treatment of chronic pancreatitis related bile duct stricture is disappointing (33%). Several studies have documented decreased stent patency over time as the patency rate dropped to 37.5% at 36 months (23, 24).
To overcome the limitation of PCSEMS related to tissue hyperplasia, fully covered self-expandable metal stent (FCSEMS) was developed. The efficacy of this type of stent in the treatment of AS following OLT is promising. The stricture resolution rate ranges from 87.5-95.5% at a follow-up period of 6-12 months. Recurrence of the stricture occurs in a very small number of patients. Stent migration is observed in many patients but no major complications have been reported (5, 25). The treatment results of chronic pancreatitis related bile duct stricture with FCSEMS, however, are less favorable.
In 2008, Cahen et al. (26) reported a small series of six patients who underwent placement of FCSEMS (Hanaro; Korea) for distal BBS from chronic pancreatitis. Stents could be removed in four patients and all achieved stricture resolution. One patient developed restenosis after 6 months. Two of six patients developed proximal stent migration resulting in unsuccessful stent removal. They subsequently underwent surgery to remove stents. A larger study by Mahajan and colleagues (6) analyzed the efficacy and complication rate of FCSEMS with and anchoring fin (Viabil; Conmed, USA) in 44 patients with BBS. In this study, nine patients had AS. The median time of SEMS placement was 3.3 months. Resolution of biliary stricture was demonstrated in 34 of 41 patients (83%), including all nine cases of AS after a median follow-up time of 3.8 months after stent removal. Chronic pancreatitis related stricture, however, had a lower resolution rate. The authors commented on the difficulty during stent removal due to the anchoring fin. In addition, bile duct injury after stent extraction was observed in 19 patients by choledochoscopy. Subsequently, Sauer et al. (15) reported their 8-year experience of the temporary CSEMS in 121 patients with BBS. This retrospective study compared the treatment results between FCSEMS and PCSEMS. FCSEMS were placed in 45% of the patients while PCSEMS were placed in 55%. The stents remained in place for a mean of 165 ± 202 days. The mean follow-up after stent removal was 735 ± 577 days. Overall stricture resolution was achieved in 63%; only 50% in chronic pancreatitis compared with 71% for other etiologies. A 20% complication rate was reported due to stent placement and 12% had complications during stent removal. Stent migration occurred in 16% of the patients. To facilitate stent removal, Wallflex stents (Boston Scientific) with flared ends and distal loop for stent retrieval were introduced.
Brijbassie et al. (16) conducted a multicenter retrospective study of 59 patients with chronic pancreatitis using Wallflex stents. Stents were removed and stricture resolution rate was 88.9% demonstrated by imaging, 91.4% documented by laboratory findings, 78.5% based on symptoms, and 85.7% by direct visualization by choledochoscopy. Minor complications included abdominal pain, pancreatitis, and stent migration.
Even though the data on CSEMS in the treatment of BBS is encouraging, the balance between its benefit of stricture resolution rate and risks including migration, infection, pancreatitis, and inability to remove stents should be strongly considered before replacing plastic stents with FCSEMS for treatment of BBS in routine practice. In a systematic review of plastic stents and metal stents for extrahepatic biliary strictures, the overall clinical success rate was 60% for single plastic stent, 94% for multiple plastic stents, and 79% for uncovered SEMS (27). Currently, comparative data on the efficacy of multiple plastic stents and covered SEMS in benign biliary stricture is lacking and therefore prospective studies are required.
The weaknesses of FCSEMS are stent migration, duodenal reflux, tissue hyperplasia, and ease of removal. These issues should be addressed in the development of stent in order to strengthen stent quality. To minimize stent migration, the concept of flared ends and anchoring flap has been introduced. Tringali et al. (28) performed a study using a nitinol covering (Niti-S; Taewoong Medical) with unflared and flared ends in 17 patients with common bile duct strictures secondary to chronic pancreatitis. After 6 months of placement and 3 years of follow-up, the flared ends had superior efficacy (87% vs. 43%) and less migration rate (57% vs. 50%). Park et al. (7) compared two types of FCSEMS; one with four anchoring flaps (AF) at the proximal end and flared distal end, and the other had flared end (FE) at both proximal and distal parts without AF, in 43 patients with BBS. After a median stent placement period of 6 months, no patients in the AF group and 33% of patients in the FE group had stent migration (p = 0.004). The removal rate of the FCSEMSs was 100% in both groups without difficulty. Immediate improvement of biliary stricture was 91% in the AF group and 88% in the FE group. During the follow-up period (median 4 months) after stent removal or migration, 16% had recurrence of the initial biliary stricture. Importantly, none of post surgical BBSs developed recurrence. The authors concluded that the AF design may be superior to the FE, with regard to the antimigration effect for benign biliary stricture and BBS from surgery had an excellent response.
Recently, a retrievable polytetrafluoroethylene (PTFE)-FCSEMS with a ball type wire mesh at the distal end (Bumpy stent, diameter 10 mm; Taewoong Medical), was designed for anti-migration and anti-reflux (29). The design to improve the ease of stent removal has also been developed by adding proximal retrievable loop allowing the stent to be pulled inside-out more easily during removal. Researchers from the Netherlands under the leadership of J.W. Poley are currently conducting a prospective study assessing a new type of fully-covered, self-expandable metal stent with a proximal retrieval lasso for the treatment of benign biliary strictures. With a better understanding of stent physics, research is actively being conducted to achieve an optimal quality of FCSEMS with the goal to improve treatment outcomes of patients suffering from BBS.
Stent migration has been a major obstacle of FCSEMS. It is hoped that the newer stent designs will decrease this problem. Acute pancreatitis is another well documented complication. Although most cases of pancreatitis are mild, SEMS results in higher rate of acute pancreatitis when compared to plastic stents (7.3% vs. 1.3%). The proposed mechanism for this observation is that the CSEMS may have higher chance of causing pancreatic orifice occlusion given its larger diameter (30). Independent predictors of post ERCP pancreatitis (PEP) are young age, previous history of PEP, and pancreatic duct injection. Acute cholecystitis and cholangitis can occur as both early and late complications if the cystic duct insertion is occluded (31). The reported rate of cholecystitis after PCMS placement ranges between 2.9% and 12% (32, 33).
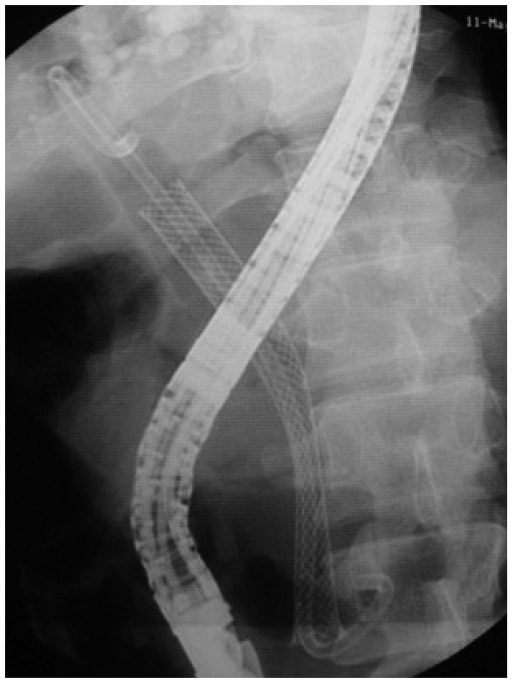
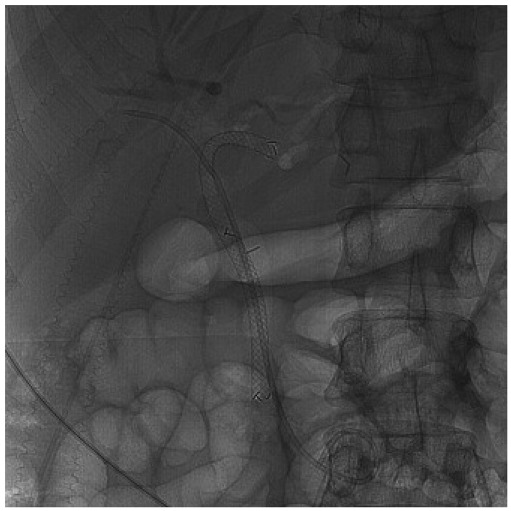
To prevent stent migration, insertion of a double pigtail stent as stent-in stent or through the SEMS mesh has been proposed (8, 34) (Fig. 2). However, there has not been a randomized control study to compare the results of this strategy. One other concern raised by experts is placing FCSEMS in the hilar stricture due to the theoretical risk of creating sub-segmental occlusion of the contralateral intrahepatic ducts by the stent membrane. This issue may be addressed by placing a plastic stent in the contralateral intrahepatic ducts to facilitate bile flow before placing the fully covered SEMS. The authors have successfully placed a 6 × 120 mm FCSEMS into the left intrahepatic duct along with a 7 French plastic stent to the right system in a case of benign biliary stricture (Fig. 3). Poley et al. (35) also reported a series of two patients who underwent placement of an intrahepatically deployed FCSEMS in conjunction with a contralateral 10 French plastic stent for 4-5 months. Both strictures resolved, and there were no signs of a recurrent stricture after a 9-month follow-up. No complications were reported.
Besides the previously mentioned complications including an increased risk of stent migration, pancreatitis, cholecystitis, and potential difficulty in stent removal, cost of FCSEMS could be a major limiting factor in a routine use of this stent. The cost effectiveness ratio (ICER) is the best way to determine the selection. It requires a calculation of stent cost, number of ERCP sessions, and the cost for additional ERCPs. The selected intervention can be determined as cost effective if its ICER is less expensive than having additional procedure(s). Currently, this issue has not been assessed. However, it has been estimated that SEMS placement may be cost-effective if the total cost of additional ERCPs for plastic stent upsizing is more expensive than US$2100. This estimate was adopted of the results from previous study on SEMS treatment for recurrent stent occlusion in patients with malignant biliary stricture (36).
Most bile leaks can be managed successfully with plastic stents with or without biliary sphinterotomy. Therefore, the role of SEMS in this condition is limited. CSEMS may be beneficial in a select group of patients who fail conventional endoscopic therapy. In one study, PCSEMS (Wallstent) were placed in 16 patients who failed plastic stent placement and were left in place for a median duration of 3 months. All except one patient had complete resolution of leak and stent migration was responsible for treatment failure (2). Wang et al. (37) reported the use of the Viabil stent in 13 patients with complexed bile leaks. Complete resolution was observed in all with complications included a stricture below the confluence in two patients. Hwang et al. (29) also reported a successful treatment in patients who had refractory bile leaks with a newly designed retrievable PTFE-FCSEMS with a ball type wire mesh at the distal end. The data on usefulness of FCSEMS in treatment of biliary leaks is currently limited.
In conclusion, FCSEMS may be a reasonably good alternative treatment option for difficult BBS and bile leaks. The current data, however, is lacking to demonstrate the superiority of FCSEMS over plastic stent placement. Randomized controlled studies assessing stent efficacy, complications, and cost-effectiveness are needed before a routine use of this modality in BBS and bile leaks can be recommended. The authors propose that FCSEMS should be considered in selected circumstances including refractory benign biliary stricture and difficult to treat bile duct stricture from chronic pancreatitis.
References
1. Perri V, Familiari P, Tringali A, Boskoski I, Costamagna G. Plastic biliary stents for benign biliary diseases. Gastrointest Endosc Clin N Am. 2011; 21:405–433. viiiPMID: 21684462.

2. Bakhru MR, Kahaleh M. Expandable metal stents for benign biliary disease. Gastrointest Endosc Clin N Am. 2011; 21:447–462. viiiPMID: 21684464.

3. Kuo MD, Lopresti DC, Gover DD, Hall LD, Ferrara SL. Intentional retrieval of viabil stent-grafts from the biliary system. J Vasc Interv Radiol. 2006; 17:389–397. PMID: 16517789.

4. Cahen DL, van Berkel AM, Oskam D, Rauws EA, Weverling GJ, Huibregtse K, et al. Long-term results of endoscopic drainage of common bile duct strictures in chronic pancreatitis. Eur J Gastroenterol Hepatol. 2005; 17:103–108. PMID: 15647649.

5. Traina M, Tarantino I, Barresi L, Volpes R, Gruttadauria S, Petridis I, et al. Efficacy and safety of fully covered self-expandable metallic stents in biliary complications after liver transplantation: a preliminary study. Liver Transpl. 2009; 15:1493–1498. PMID: 19877248.

6. Mahajan A, Ho H, Sauer B, Phillips MS, Shami VM, Ellen K, et al. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video). Gastrointest Endosc. 2009; 70:303–309. PMID: 19523620.

7. Park do H, Lee SS, Lee TH, Ryu CH, Kim HJ, Seo DW, et al. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc. 2011; 73:64–70. PMID: 21184871.

8. Hu B, Gao DJ, Yu FH, Wang TT, Pan YM, Yang XM. Endoscopic stenting for post-transplant biliary stricture: usefulness of a novel removable covered metal stent. J Hepatobiliary Pancreat Sci. 2011; 18:640–645. PMID: 21643818.

9. Hall JG, Pappas TN. Current management of biliary strictures. J Gastrointest Surg. 2004; 8:1098–1110. PMID: 15585398.

10. Shanbhogue AK, Tirumani SH, Prasad SR, Fasih N, McInnes M. Benign biliary strictures: a current comprehensive clinical and imaging review. AJR Am J Roentgenol. 2011; 197:W295–W306. PMID: 21785056.

11. Larghi A, Tringali A, Lecca PG, Giordano M, Costamagna G. Management of hilar biliary strictures. Am J Gastroenterol. 2008; 103:458–473. PMID: 18028506.

12. Kaffes AJ, Hourigan L, De Luca N, Byth K, Williams SJ, Bourke MJ. Impact of endoscopic intervention in 100 patients with suspected postcholecystectomy bile leak. Gastrointest Endosc. 2005; 61:269–275. PMID: 15729238.

13. Kahaleh M, Behm B, Clarke BW, Brock A, Shami VM, De La Rue SA, et al. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video). Gastrointest Endosc. 2008; 67:446–454. PMID: 18294506.

14. Chaput U, Scatton O, Bichard P, Ponchon T, Chryssostalis A, Gaudric M, et al. Temporary placement of partially covered self-expandable metal stents for anastomotic biliary strictures after liver transplantation: a prospective, multicenter study. Gastrointest Endosc. 2010; 72:1167–1174. PMID: 20970790.

15. Sauer BG, Regan KA, Srinivasan I, Shami VM, Rehan ME, Ellen K, et al. Temporary placement of covered self-expandable metal stents (CSEMS) in benign biliary strictures (BBS): eight years of experience. Gastrointest Endosc. 2010; 71:AB110–AB111.
16. Brijbassie A, Stevens PD, Sethi A, Sejpal DV, Raijman I, Loren DE, et al. Use of fully covered self expanding metal stents (FCSEMS) in the management of benign biliary diseases (BBD). Gastrointest Endosc. 2010; 71:AB298.
17. Pascher A, Neuhaus P. Bile duct complications after liver transplantation. Transpl Int. 2005; 18:627–642. PMID: 15910286.

18. Bismuth H. Blumgart LH, editor. Postoperative stricture of the bile duct. The biliary tract. 1982. Edinburgh: Churchill Livingstone;p. 209–218.
19. Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995; 180:101–125. PMID: 8000648.
20. Draganov P, Hoffman B, Marsh W, Cotton P, Cunningham J. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc. 2002; 55:680–686. PMID: 11979250.

21. Vandenbroucke F, Plasse M, Dagenais M, Lapointe R, Lêtourneau R, Roy A. Treatment of post liver transplantation bile duct stricture with self-expandable metallic stent. HPB (Oxford). 2006; 8:202–205. PMID: 18333277.

22. Tee HP, James MW, Kaffes AJ. Placement of removable metal biliary stent in post-orthotopic liver transplantation anastomotic stricture. World J Gastroenterol. 2010; 16:3597–3600. PMID: 20653071.

23. Cantù P, Hookey LC, Morales A, Le Moine O, Devière J. The treatment of patients with symptomatic common bile duct stenosis secondary to chronic pancreatitis using partially covered metal stents: a pilot study. Endoscopy. 2005; 37:735–739. PMID: 16032492.

24. Behm B, Brock A, Clarke BW, Ellen K, Northup PG, Dumonceau JM, et al. Partially covered self-expandable metallic stents for benign biliary strictures due to chronic pancreatitis. Endoscopy. 2009; 41:547–551. PMID: 19533560.

25. García-Pajares F, Sánchez-Antolín G, Pelayo SL, Gómez de la Cuesta S, Herranz Bachiller MT, Pérez-Miranda M, et al. Covered metal stents for the treatment of biliary complications after orthotopic liver transplantation. Transplant Proc. 2010; 42:2966–2969. PMID: 20970584.

26. Cahen DL, Rauws EA, Gouma DJ, Fockens P, Bruno MJ. Removable fully covered self-expandable metal stents in the treatment of common bile duct strictures due to chronic pancreatitis: a case series. Endoscopy. 2008; 40:697–700. PMID: 18704837.

27. van Boeckel PG, Vleggaar FP, Siersema PD. Plastic or metal stents for benign extrahepatic biliary strictures: a systematic review. BMC Gastroenterol. 2009; 9:96. PMID: 20017920.

28. Tringali A, Familiari P, Mutignani M, Perri V, Costamagna G. Self-expandable, removable, fully covered metal stents to dilate common bile duct strictures secondary to chronic pancreatitis: preliminary results. Gastrointest Endosc. 2010; 71:AB169–AB170.
29. Hwang JC, Kim JH, Yoo BM, Lim SG, Kim JH, Kim WH, et al. Temporary placement of a newly designed, fully covered, self-expandable metal stent for refractory bile leaks. Gut Liver. 2011; 5:96–99. PMID: 21461081.

30. Coté GA, Kumar N, Ansstas M, Edmundowicz SA, Jonnalagadda S, Mullady DK, et al. Risk of post-ERCP pancreatitis with placement of self-expandable metallic stents. Gastrointest Endosc. 2010; 72:748–754. PMID: 20630513.

31. Ho H, Mahajan A, Gosain S, Jain A, Brock A, Rehan ME, et al. Management of complications associated with partially covered biliary metal stents. Dig Dis Sci. 2010; 55:516–522. PMID: 19267200.

32. Bezzi M, Zolovkins A, Cantisani V, Salvatori FM, Rossi M, Fanelli F, et al. New ePTFE/FEP-covered stent in the palliative treatment of malignant biliary obstruction. J Vasc Interv Radiol. 2002; 13:581–589. PMID: 12050298.

33. Hausegger KA, Thurnher S, Bodendörfer G, Zollikofer CL, Uggowitzer M, Kugler C, et al. Treatment of malignant biliary obstruction with polyurethane-covered Wallstents. AJR Am J Roentgenol. 1998; 170:403–408. PMID: 9456954.

34. Ridtitid W, Rerknimitr R, Amornsawadwattana S, Ponauthai Y, Kullavanijaya P. Stent-in-stent through a side hole to prevent biliary metallicstent migration. World J Gastrointest Endosc. 2011; 3:64–66. PMID: 21455345.

35. Poley JW, van Tilburg AJ, Kuipers EJ, Bruno MJ. Breaking the barrier: using extractable fully covered metal stents to treat benign biliary hilar strictures. Gastrointest Endosc. 2011; 74:916–920. PMID: 21821252.

36. Ridtitid W, Rerknimitr R, Janchai A, Kongkam P, Treeprasertsuk S, Kullavanijaya P. Reply to Dr. Viroj Wiwanikit. Surg Endosc. 2011; [Epub ahead of print].

37. Wang AY, Ellen K, Berg CL, Schmitt TM, Kahaleh M. Fully covered self-expandable metallic stents in the management of complex biliary leaks: preliminary data - a case series. Endoscopy. 2009; 41:781–786. PMID: 19693751.

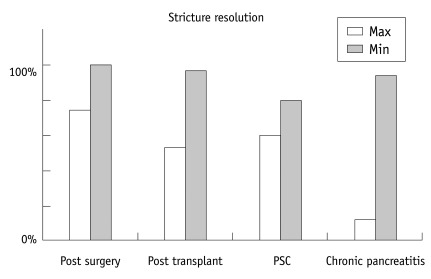
Fig. 1
Results of pancreatic stent treatment in benign biliary striture of different etiologies. Maximum result in post-transplant stricture was found in anastomotic stricture whereas minimum result was found in non-anastomotic stricture. PSC = Primary sclerosing cholangitis, Max = Maximum percentage of response, Min = Minimum percentage of response




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download