Abstract
Major vascular complications related to pancreatitis can cause life-threatening hemorrhage and have to be dealt with as an emergency, utilizing a multidisciplinary approach of angiography, endoscopy or surgery. These may occur secondary to direct vascular injuries, which result in the formation of splanchnic pseudoaneurysms, gastrointestinal etiologies such as peptic ulcer disease and gastroesophageal varices, and post-operative bleeding related to pancreatic surgery. In this review article, we discuss the pathophysiologic mechanisms, diagnostic modalities, and treatment of pancreatic vascular complications, with a focus on the role of minimally-invasive interventional therapies such as angioembolization, endovascular stenting, and ultrasound-guided percutaneous thrombin injection in their management.
It has been estimated that there are more than 210000 admissions for acute pancreatitis and more than 56000 hospitalizations for chronic pancreatitis in the United States each year (1). Overall, the incidence of pancreatitis has been increasing gradually (2). The severe form of acute pancreatitis, including necrotizing pancreatitis, occurs in about 20-30% of all patients with this disease, resulting in a protracted clinical course, high incidence of local complications, and high mortality rate (3).
In the literature, major vascular complications of pancreatitis occur with a frequency of 1.2-14%, with a greater incidence seen in chronic pancreatitis (7-10%) than acute pancreatitis (1-6%) (4, 5). The overall mortality rate due to hemorrhage in acute pancreatitis has been reported to reach ranges as high as 34-52%, and is significantly higher than in cases of patients without bleeding (5). However, deaths in pancreatitis patients often occur due to multi-organ failure and sepsis (from exacerbation of a patient's clinical condition), rather than from the bleeding event directly (5).
Although rare, direct vascular injuries are the most feared complications as they can cause rapid blood loss and clinical deterioration. Most commonly, these injuries lead to pseudoaneurysm formation, where blood collects in a contained peritoneal space or organ. Less commonly, there occurs arterial rupture into a pancreatic pseudocyst (converting it into a pseudoaneurysm) and rarely, arterial rupture into the gastrointestinal (GI) tract.
Indirect causes of bleeding primarily involve the GI tract and are due to peptic ulcer disease, stress gastritis, gastroesophageal varices, or Mallory-Weiss tears. They are more prevalent compared to direct vascular injuries in the setting of pancreatitis, with an overall reported incidence of 22% (6). A higher rate of peptic ulcer disease in patients with pancreatitis, as compared to the general population, is well established (6). In general, vascular complications, both direct and indirect, have to be dealt with as an emergency, utilizing a multidisciplinary approach of angiography, endoscopy or surgery.
In this article, we discuss the pathophysiologic mechanisms leading to pancreatic vascular complications, and the common modalities utilized in their diagnosis such as ultrasound, CT angiography, and catheter angiography. Various therapeutic options will also be discussed, including the primary role played by interventional radiology in the treatment of vascular complications.
The risk factors for major vascular complications include necrotizing pancreatitis, multi-organ failure, sepsis, and pancreatic fluid-collections such as abscesses, pseudocysts or walled-off necrosis (Fig. 1). Previous pancreatic necrosectomy, long-term anticoagulation therapy, and underlying vasculitis also elevate the probability of developing this complication (5).
One of the causes of vessel injury is severe pancreatic inflammation and necrosis, which results in the local spread of exocrine proteolytic and lipolytic enzyme-rich fluids. These fluids cause weakening and elastolytic erosions of the vessel wall, which may result in the formation of a pseudoaneurysm (if there is continued bleeding into a contained space or organ), contained hematoma (if the pseudoaneurysm becomes thrombosed or active extravasation stops), or frank intraperitoneal hemorrhage (if the pseudoaneurysm ruptures) (4).
Another possible mechanism of vessel injury occurs due to iatrogenic causes after pancreatic surgery. Pancreaticoduodenectomy (Whipple procedure) and pancreatic necrosectomy are especially prone to post-operative vascular complications, with multiple reported cases of pseudoaneurysm formation and GI hemorrhage (Fig. 2) (4, 7). Too aggressive of an intra-operative technique may injure the vessel wall directly, which combined with the proteolytic digestive enzymes in the local environment may lead to vessel rupture. Surgical and percutaneous drains, placed for long-term lavage and treatment of pancreatic fluid-collections, also elevate the risk of injuring the vasculature. This is due to the mechanical irritation and trauma caused by the tubes on inflamed and denuded vessels. Post-surgical packing of residual spaces with gauze or sponges may also cause vascular erosion in a similar manner (4). Furthermore, bleeding may occur from the dehiscence of vascular stumps or anastomotic sites post-operatively. Pseudoaneurysm formation after pancreatic surgery is often a late complication, usually occurring several days to weeks after the operation (8).
Severe hemorrhage may also complicate long-standing pseudocysts, converting them to pseudoaneurysms. These can become large in size and cause persistent compression, ischemia, and elastolytic degradation of the vessel wall due to their enzymatic content (Fig. 3). Through a similar mechanism, long-standing abscesses have also been reported as converting into pseudoaneurysms, but are more often observed weeks to months after a severe bout of pancreatitis (9).
Pseudoaneurysms may become pulsatile and eventually rupture into the peritoneal cavity, retroperitoneum, or adjacent structures such as bowel (presenting as massive GI hemorrhage) (10) and pancreatic duct of Wirsung (presenting as hemosuccus pancreaticus) (4). Intrasplenic and subcapsular hematoma formation and splenic rupture have also been reported in the literature after the development of pseudoaneurysms and arterial bleeding. Additionally, arterial compromise in the mesentery, as a result of bleeding events, can lead to segmental colonic and small bowel ischemia and infarction (9).
The pancreas is intimately associated with the splenic vein, inferior mesenteric vein, superior mesenteric vein and portal vein. Splenic vein thrombosis, caused by local inflammation or compression from an adjacent peripancreatic fluid-collection, may result in isolated left-sided portal hypertension, gastroesophageal variceal formation, and eventual GI bleeding. Thus, splenic vein thrombosis is more likely to occur in the presence of pseudocysts and severe necrotizing pancreatitis (4). Complications related to portal vein involvement are also most commonly due to thrombosis; development of pancreaticoportal fistulas and portal vein ruptures are very rare with few reported cases (11).
Gastrointestinal hemorrhage due to peptic ulcers and stress gastritis may occur at any time during the course of the illness. A retrospective study evaluating a total of 123 patients by Lee et al. (12) demonstrated a high prevalence of peptic ulcer disease (52.6%) in acute pancreatitis, owing to the stressful nature of the underlying disease. Pathophysiologic mechanisms for this association include the hypovolemia present in acute pancreatitis, which results in hypoperfusion and ischemia of the gastric mucosa, leading to stress gastritis and ulceration. Also, reduced bicarbonate secretion from the inflamed pancreas causes increased acidity in the duodenal lumen, predisposing the mucosa to ulceration.
Vascular injuries are often noninvasively diagnosed with CT angiography (CTA) and occasionally with ultrasonography (US). Conventional angiography, however, remains the gold standard for the accurate diagnosis of pseudoaneurysms and arterial injuries. Currently, only anecdotal evidence supports the utility of magnetic resonance angiography in helping establish a diagnosis in this acute clinical condition; therefore, this modality will not be discussed.
CTA is a relatively fast modality that is less operator dependent, has a shorter acquisition time (≤ 1 min), and demonstrates high accuracy in diagnosing arterial injuries (13). CTA also can demonstrate the full extent of a pseudoaneurysm if partially thrombosed, and its effect on the adjacent viscera. Immediate post-processing with maximum intensity projections and 3-D reconstructions (Fig. 2) with vessel analysis can prove invaluable in pre-procedural planning, whether treatment involves angiography or surgery. On the other hand, exposure to ionizing radiation and iodinated contrast material may confer additional risks to the patient, although, in most instances, the benefits of this modality far outweigh the risks.
Doppler US helps establish a diagnosis of pseudoaneurysm by demonstrating a characteristic "yin-yang sign" on imaging and a "to-and-fro" wave-form. However, this may also be seen in the presence of true aneurysms; therefore, additional clinical context is crucial in establishing the diagnosis. US is portable, readily available, inexpensive, relatively fast, and involves no ionizing radiation or contrast agent. The modality has been reported to have a sensitivity of 94% and a specificity of 97% in the detection of post-catheterization pseudoaneurysms, but is limited in its ability to detect deep visceral pseudoaneurysms (it does so with low sensitivity) (13). However, if a pseudoaneurysm is identified by US, thrombotic resolution after interventional treatment can often be assessed, precluding the use of a follow-up CTA (Fig. 4).
One significant advantage of angiography is the evaluation of an arterial bed in real-time, which includes accurately identifying the supplying vessel and assessing collateral circulation to determine the expendability of the parent artery. This is important for planning treatment, which is discussed below. Other smaller pseudoaneurysms not identified by US or CTA may also be identified by catheter angiography. Lesions such as true aneurysms, arteriovenous fistulas, and vascular malformations, which can have a similar appearance to pseudoaneurysms on other imaging modalities, may also be accurately resolved. Detailed characteristics of pseudoaneurysms obtained during angiography, including parent vessel size and aneurysm neck size, further aid in planning treatment (13). The modality also proves useful in localizing the bleeding site in those patients for whom endoscopy or surgery was unsuccessful as the initial treatment (14).
Balachandra and Siriwardena, who evaluated published series of cases with vascular complications of pancreatitis, reported that of 173 patients undergoing abdominal angiography, 94% showed either a source of bleeding or a false aneurysm. Angiography had a sensitivity of 100% for the detection of arterial bleeding in patients with pancreatic pseudoaneurysms (15). Therefore, catheter angiography remains a powerful diagnostic tool with added therapeutic potential if indicated.
However, this remains an invasive modality, conferring additional risk of vascular complications such as dissection, pseudoaneurysm rupture, or vessel thrombosis. Access site hematomas and femoral artery pseudoaneurysms are also associated with catheterization. Ionizing radiation and iodinated contrast material present their own inherent risks to the patient during possibly lengthy interventions (13). Another limitation of the modality is that it does not accurately help assess the size and full extent of a pseudoaneurysm containing thrombus, although this lack of information rarely affects treatment decisions or clinical outcomes. Finally, failure to detect bleeding by angiography may be attributed to intermittent bleeding or the fact that bleeds may have a venous rather than arterial origin (9).
The management of pancreatic pseudoaneurysms depends on factors such as hemodynamic stability, coagulation status, and source of bleeding. Their natural course is unpredictable; some undergo spontaneous thrombosis while others spontaneously rupture. Regardless of their size, the risk of spontaneous rupture of extra-organic visceral pseudoaneurysms is very high and the mortality rate of such ruptures in morbid post-surgical patients has been reported to approach 100% (13). Therefore, many authors believe that definitive treatment should be administered as soon as possible (13). The most common splanchnic artery reported to develop a pseudoaneurysm is the splenic artery (Figs. 1, 5) followed by the hepatic artery (Fig. 2) (16).
Endovascular therapy remains the first-line option for known arterial bleeding (preferably in a hemodynamically stable patient) and is considered a safe and effective modality for treating visceral pseudoaneurysms. The reported success rate of endovascular therapy is high, ranging from 79-100%, with recurrent bleeding rates ranging from 18-37% (15, 17). However, data relating to long-term maintenance of hemostasis after successful intervention is limited. The overall mortality related to angiographic failure or a complication was reported to be 14% in patients with chronic pancreatitis. In addition, there was a lower requirement for blood transfusions and a shorter length of hospital stay following endovascular intervention compared to surgery (18).
US-guided percutaneous thrombin injection into visceral pseudoaneurysms has also been demonstrated as a valuable alternative in cases of angiographic failure or situations when endovascular therapy is not technically feasible. If visualized by US, thrombin can be injected directly into the site of blood extravasation and evaluated in real-time for cessation of flow. This procedure has proved to be valuable in the treatment of iatrogenic false aneurysms involving femoral artery access sites, with a recurrence rate of 2% and a rate of distal embolization of 0.95% (19, 20). The effectiveness of this procedure in the treatment of visceral pseudoaneurysms is not well established; however, numerous case reports in the literature have documented the technique's efficacy (19-21). Sparrow et al. (21) described a case of a 3 cm pancreatic head pseudoaneurysm that was successfully treated using ultrasound-guided thrombin injections due to their inability to catheterize the feeding vessel on angiography.
Surgical treatment of a pseudoaneurysm is indicated by patient instability, lack of angiographic availability, or angiographic failure. However, there are limitations with surgery. The injured vessel usually lies deep within the pancreatic parenchyma or pseudocyst with a location that cannot be accurately defined (5). Due to the friability of pancreatic tissue and pseudocyst wall, oversewing of a presumed bleeding site will usually be unsuccessful in providing adequate hemostasis or preventing future hemorrhage (22). Additionally, dense adhesions created from episodes of pancreatic inflammation grossly distort normal anatomy and contribute to operative complexity (17).
If proximal ligation of the bleeding vessel is not possible, then pancreatic resection is the only alternative. The mortality for pancreatic resection due to a bleeding lesion is 43% in the pancreatic head and 16% in the pancreatic body and tail (15). A Whipple procedure is usually performed for bleeding pseudoaneurysms near the pancreatic head, and a distal pancreatectomy with or without splenectomy is indicated for lesions around the pancreatic body or tail (22).
There is also an increased risk of delayed bleeding post-pancreatic surgery, with an overall reported prevalence of 5-12% (Fig. 2) (7). A high-risk, re-exploration laparotomy is usually required if re-bleeding were to occur; therefore, catheter angiography proves its utility once again in this scenario. In a recent retrospective analysis of 1669 consecutive pancreatic resections, Yekebas et al. (7) concluded that angiography is the first diagnostic and therapeutic tool for delayed post-pancreatectomy hemorrhage, whether bleeding occurs intraluminally or extraluminally.
Other causes of bleeding such as peptic ulcers, stress gastritis, gastroesophageal varices, and Mallory-Weiss tears are mostly treated with a combination of medical management and endoscopic therapy. In instances when endoscopic therapy fails, angiography again may be performed to identify the source of hemorrhage, with surgical therapy as a treatment of last resort. Failure of endoscopic therapy may be related to gastric fundal varices, intermittent bleeding through the papilla, or a bleeding source beyond the reach of the endoscope (for example, small intestinal varices or pseudoaneurysm rupture into the intestine) (9).
Initial diagnostic angiography must first be performed for of both the celiac axis and superior mesenteric artery (SMA). These aortic branches are cannulated commonly with a curved catheter such as the Cobra. During difficult cannulations due to acutely angulated branches, a reverse curved catheter such as the SOS Omni (AngioDynamics, Queensbury, NY, USA) or a Simmons catheter may be needed. Anteroposterior and oblique projection angiograms are often obtained of the celiac bed or SMA to help delineate the complex vascular anatomy. Superselection of smaller arterial branches may then be performed if required, with the aid of microcatheters and microwires.
For arterial systems with extensive collateral circulation, such as the celiac axis, aggressive coil embolization can be performed with low risk of distal ischemia. Proximal SMA branches such as the inferior pancreaticoduodenal artery also have adequate collateral circulation and may be expendable (Fig. 6); however, inadvertent embolization of an intestinal branch may result in small bowel infarction. Therefore, care has to be taken when dealing with the SMA.
Metallic coil embolization of expendable arteries (that is arteries with an extensive collateral circulation) is preferable distal and proximal to the site of arterial extravasation (the so-called "sandwich" technique), thereby preventing backflow from the collateral circulation (Figs. 1, 3-6) (13, 23). In situations where the artery is inexpendable, careful evaluation of the pseudoaneurysm neck size should be made. If the neck is narrow, the pseudoaneurysm may be embolized with catheter-directed delivery of coils into the sac itself or excluded by placement of a covered stent-graft (Fig. 2). If the neck is wide, a covered stent-graft alone may provide sufficient exclusion of the pseudoaneurysm; however, placing coils into the sac poses a significant risk of the coils backing out into the parent artery (13). Another option is using a stent-cage, where a non-covered stent-graft is deployed across the aneurysm neck, followed by catheter-directed coil embolization through the stent interstices. The stent acts as a mechanical barrier, confining or "jailing" the coils within the pseudoaneurysm sac (13).
However, there are times when an inexpendable artery has to be sacrificed if the patient becomes hemodynamically unstable or if stent placement and coil embolization become technically challenging. For example, small or tortuous arterial branches are difficult to access, even with the aid of microcatheters. Thus, coils may have to be deployed into the parent artery, proximal to the arterial injury, with hopes of sufficient hemostasis (Fig. 5).
Permanent occlusion of small or tortuous vascular branches and multiple collateral sources may also be accomplished using liquid embolic agents such as N-butyl cyanoacrylate (NBCA; TRUFILL Liquid Embolic System, Cordis Neurovascular, Miami Lakes, FL, USA), and ethylene vinyl alcohol copolymer (Onyx; ev3, Plymouth, MN, USA). These materials have the advantage of low viscosity, allowing a single injection to simultaneously fill numerous downstream vascular channels before polymerizing into a hard cast (8). In an analysis of 12 patients treated with liquid embolic agents for pseudoaneurysms related to pancreatitis or pancreatectomy, Izaki et al. (8) reported a technical success rate of 100%, no recurrent bleeding, and no major complications. Difficulties associated with the use of these agents include catheter adherence to the material from early polymerization and non-target embolization.
Another option for occluding small or tortuous vascular branches is the use of Gelfoam slurry (Ethicon, Somerville, NJ, USA). Gelfoam remains a relatively safe but temporary embolic material, with the embolized vessel usually recanalizing in a few weeks. Often times, this temporizing measure provides adequate time for the vascular injury to heal (23). Also, similar to glue injection, multiple efferent vascular branches can be simultaneously occluded with Gelfoam.
Placement of an Amplatzer vascular plug (AVP) (St. Jude Medical, St. Paul, MN, USA) can also provide fast and complete occlusion of a large artery feeding a pseudoaneurysm. The main advantages of this device are its detachability and ability to occlude larger sized vessels (such as the splenic artery) faster than with the placement of multiple coils. However, these devices are problematic for smaller-sized and tortuous vessels due to their requirement for larger delivery systems and limited maneuverability. Newer generation AVP devices require smaller delivery systems (4-7 Fr sheaths), are more maneuverable, and are more thrombogenic (23).
After intervention, it is recommended that a completion celiac and SMA angiogram be performed to ensure that there are no other collateral vessels supplying the pseudoaneurysm or endoleaks complicating stent-graft deployment. Any supplementary branches must be selectively embolized and significant endoleaks treated by performing balloon angioplasty of the stent-graft or deploying an additional overlapping stent. Sometimes, small residual endoleaks may be left alone and followed using non-invasive imaging. These tend to undergo thrombosis spontaneously due to their low rate of blood flow.
Complications associated with angiographic embolization, such as intraprocedural rupture of the pseudoaneurysm and delayed reconstitution of arterial flow, result in the failure of treatment. However, these complications are rare and are mostly unrelated to angiographic technique (13). Non-target coil embolization and maldeployment of a stent-graft, resulting in stent migration or stent infolding, are technique-related and may occur due to inappropriate vessel-sizing or device selection. Furthermore, continued decreases in hemoglobin and hematocrit post-procedure should prompt urgent re-evaluation of the patient with the use of non-invasive imaging modalities or repeat angiography. Figure 5 describes a case where a small pseudoaneurysm arising from a branch of the distal pancreatic artery was discovered days after the patient had been treated for a splenic artery pseudoaneurysm related to pancreatitis.
Finally, arterial embolization may sometimes result in solid organ ischemia and subsequent development of an organic abscess (Fig. 1) (24). Introduction of exogenous bacteria by percutaneous means, retrograde transport of enteric pathogens via a reversed portal flow, immunosuppression, and biliary ischemia are reported mechanisms of abscess formation in the liver and spleen (24, 25). Intraparenchymal and intraperitoneal abscesses can often be percutaneously or surgically drained.
Major vascular complications in pancreatitis are rare, and have to be treated as an emergency due to their potentially fatal consequences. The most feared of these is arterial rupture with pseudoaneurysm formation. Interventional radiology remains the mainstay in the accurate diagnosis of such complications and offers effective, minimally-invasive, therapeutic options such as angioembolization, endovascular stenting, and percutaneous thrombin injection. For technical success and clinical resolution, it is essential to recognize these complications early, collaborate with gastroenterological and surgical colleagues, and carefully scrutinize US, CT, and angiographic imaging, so that the most ideal interventional treatment strategy may be developed.
Figures and Tables
Fig. 1
82-year-old patient with history of common bile duct stent placement and prior gastroduodenal coil embolization presents with severe pancreatitis and blood loss.
A. Axial CT image demonstrates walled-off pancreatic necrosis (arrowhead) and new peripancreatic pseudoaneurysm arising from splenic artery (arrow). B. Angiogram confirms active contrast extravasation from splenic artery (arrow). C. Coils are deployed in mid-portion of splenic artery (arrow) distal and proximal to site of contrast extravasation ("sandwich" technique). Also seen is common trunk of superior mesenteric artery and celiac axis (arrowhead). D. Follow-up axial CT image demonstrates interval splenic infarction and development of splenic abscess (arrow); complication related to embolization. Patient ultimately died from multi-organ failure.
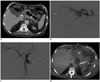
Fig. 2
Patient with severe blood loss after Whipple procedure.
A, B. 3D reconstructed CT image and angiogram demonstrate large pseudoaneurysm arising from mid-common hepatic artery (arrows). This is inexpendable artery and coil embolization of artery is contraindicated due to possibility of end-organ ischemia. C. Thus, covered stent-graft was deployed across site of arterial injury with complete exclusion of pseudoaneurysm. Another strategy would have been to coil embolize pseudoaneurysm directly if stent-graft deployment was not feasible. (Case courtesy of Dr. Bart Dolmatch, University of Texas Southwestern, Dallas, TX, USA).
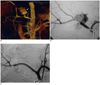
Fig. 3
32-year-old patient with chronic pancreatitis who had previously undergone splenectomy for splenic vein thrombosis presents with abdominal pain and blood loss.
A. Axial CT image, one year prior, demonstrates moderate-sized pseudocyst in the region of pancreatic body (arrow). B. Axial CT image, one year later, shows interval conversion of this long-standing pseudocyst into pseudoaneurysm (arrow). C. Left gastric arteriogram reveals large pseudoaneurysm arising from proximal aspect of inferior branch (arrow). D. Using microcatheter, coil embolization is performed distal and proximal to arterial injury ("sandwich" technique) (arrowheads).
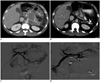
Fig. 4
Patient with chronic pancreatitis who presents with abdominal pain and blood loss.
A. Axial CT angiography image reveals large hemorrhagic pseudocyst adjacent to pancreatic head (arrow) with mass effect causing biliary dilation. Active contrast extravasation is seen anteriorly (arrowhead). Another large pseudocyst is also present in right posterior pararenal space causing mass effect upon right kidney. B. Celiac angiogram confirms contrast extravasation from gastroduodenal artery (GDA) (arrow). C. A microcatheter is advanced distal to arterial injury in GDA and coil embolization performed distal to proximal (arrowheads). D. Follow-up targeted ultrasound confirms thrombosis of pseudoaneurysm.
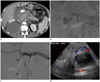
Fig. 5
Patient with history of pancreatitis who presents with blood loss.
A. Angiogram demonstrates distal splenic arterial pseudoaneurysm (arrow). B. Pseudoaneurysm was coil embolized using microcatheter with complete exclusion (arrowheads). C. However, patient continued to bleed during his admission and repeat catheter angiography discovered additional small pseudoaneurysm arising from branch of distal pancreatic artery (arrow). D. Using microcatheter technique, single coil was deployed in this arterial branch proximal to pseudoaneurysm with adequate exclusion (arrowhead).
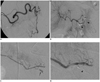
Fig. 6
46-year-old patient with history of chronic pancreatitis and prior splenic embolization, who presented with abdominal pain and blood loss.
A. Axial CT angiography image demonstrates bilobed pseudoaneurysm arising from inferior pancreaticoduodenal arcade near pancreatic head (arrow). B. Superior mesenteric artery angiogram confirms presence of pseudoaneurysm arising from branch of inferior pancreaticoduodenal arcade (arrow). Prior splenic embolization coils are also seen (arrowhead). C. Coil embolization is performed of this branch distal and proximal to arterial injury (arrowheads) using microcatheter technique with complete exclusion of pseudoaneurysm. Collateral circulation supplies superior pancreaticoduodenal, gastroduodenal, and proper hepatic arteries.
References
1. Affronti J. Chronic pancreatitis and exocrine insufficiency. Prim Care. 2011. 38:515–537. ix
2. Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006. 354:2142–2150.
3. Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology. 2002. 223:603–613.
4. Flati G, Andrén-Sandberg A, La Pinta M, Porowska B, Carboni M. Potentially fatal bleeding in acute pancreatitis: pathophysiology, prevention, and treatment. Pancreas. 2003. 26:8–14.
5. Sharma PK, Madan K, Garg PK. Hemorrhage in acute pancreatitis: should gastrointestinal bleeding be considered an organ failure? Pancreas. 2008. 36:141–145.
6. Bergert H, Dobrowolski F, Caffier S, Bloomenthal A, Hinterseher I, Saeger HD. Prevalence and treatment of bleeding complications in chronic pancreatitis. Langenbecks Arch Surg. 2004. 389:504–510.
7. Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007. 246:269–280.
8. Izaki K, Yamaguchi M, Kawasaki R, Okada T, Sugimura K, Sugimoto K. N-butyl cyanoacrylate embolization for pseudoaneurysms complicating pancreatitis or pancreatectomy. J Vasc Interv Radiol. 2011. 22:302–308.
9. Andersson E, Ansari D, Andersson R. Major haemorrhagic complications of acute pancreatitis. Br J Surg. 2010. 97:1379–1384.
10. Iwama Y, Sugimoto K, Zamora CA, Yamaguchi M, Tsurusaki M, Taniguchi T, et al. Transcatheter embolization of splenic artery pseudo-aneurysm rupturing into colon after post-operative pancreatitis. Cardiovasc Intervent Radiol. 2006. 29:133–136.
11. Charvat F, Maskova J, Belina F, Buric I, Lacman J, Fuksa Z, et al. Portal vein erosion: a rare hemorrhagic complication of acute pancreatitis treated by percutaneous stent-graft placement. J Vasc Interv Radiol. 2010. 21:411–412.
12. Lee KM, Paik CN, Chung WC, Yang JM. Association between acute pancreatitis and peptic ulcer disease. World J Gastroenterol. 2011. 17:1058–1062.
13. Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005. 25:Suppl 1. S173–S189.
14. Balachandra S, Siriwardena AK. Systematic appraisal of the management of the major vascular complications of pancreatitis. Am J Surg. 2005. 190:489–495.
15. Hsu JT, Yeh CN, Hung CF, Chen HM, Hwang TL, Jan YY, et al. Management and outcome of bleeding pseudoaneurysm associated with chronic pancreatitis. BMC Gastroenterol. 2006. 6:3.
16. Harvey J, Dardik H, Impeduglia T, Woo D, DeBernardis F. Endovascular management of hepatic artery pseudoaneurysm hemorrhage complicating pancreaticoduodenectomy. J Vasc Surg. 2006. 43:613–617.
17. Zyromski NJ, Vieira C, Stecker M, Nakeeb A, Pitt HA, Lillemoe KD, et al. Improved outcomes in postoperative and pancreatitis-related visceral pseudoaneurysms. J Gastrointest Surg. 2007. 11:50–55.
18. Udd M, Leppäniemi AK, Bidel S, Keto P, Roth WD, Haapiainen RK. Treatment of bleeding pseudoaneurysms in patients with chronic pancreatitis. World J Surg. 2007. 31:504–510.
19. Francisco LE, Asunción LC, Antonio CA, Ricardo RC, Manuel RP, Caridad MH. Post-traumatic hepatic artery pseudoaneurysm treated with endovascular embolization and thrombin injection. World J Hepatol. 2010. 2:87–90.
20. Dambrin C, Marcheix B, Birsan T, Cron C, Muscari F, Suc B, et al. Posttraumatic pseudoaneurysm of the hepatic artery: treatment with ultrasound-guided percutaneous transhepatic thrombin injection. J Trauma. 2005. 59:239–242.
21. Sparrow P, Asquith J, Chalmers N. Ultrasonic-guided percutaneous injection of pancreatic pseudoaneurysm with thrombin. Cardiovasc Intervent Radiol. 2003. 26:312–315.
22. Bender JS, Bouwman DL, Levison MA, Weaver DW. Pseudocysts and pseudoaneurysms: surgical strategy. Pancreas. 1995. 10:143–147.
23. Lopera JE. Embolization in trauma: principles and techniques. Semin Intervent Radiol. 2010. 27:14–28.
24. Sanada Y, Kondo H, Goshima S, Kanematsu M, Tanaka Y, Tokuyama Y, et al. Liver abscess after common hepatic artery embolization for delayed hemorrhage following pancreaticoduodenectomy: a case report. Case Report Med. 2010. 2010:280430.
25. Madoff DC, Denys A, Wallace MJ, Murthy R, Gupta S, Pillsbury EP, et al. Splenic arterial interventions: anatomy, indications, technical considerations, and potential complications. Radiographics. 2005. 25:Suppl 1. S191–S211.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download