Abstract
Nonvariceal upper gastrointestinal (UGI) bleeding is a frequent complication with significant morbidity and mortality. Although endoscopic hemostasis remains the initial treatment modality, severe bleeding despite endoscopic management occurs in 5-10% of patients, necessitating surgery or interventional embolotherapy. Endovascular embolotherapy is now considered the first-line therapy for massive UGI bleeding that is refractory to endoscopic management. Interventional radiologists need to be familiar with the choice of embolic materials, technical aspects of embolotherapy, and the factors affecting the favorable or unfavorable outcomes after embolotherapy for UGI bleeding.
Nonvariceal upper gastrointestinal (UGI) bleeding is defined as bleeding originating at the level of the distal esophagus, stomach, and the duodenum (proximal to the ligament of Treitz). Nonvariceal UGI bleeding results in the hospitalization of 30-100 patients per 100000 population per year, which is two-to-four times more common than lower GI bleeding (1).
The most common causes of UGI bleeding are peptic ulcer disease, benign and malignant tumors, gastritis, arteriovenous malformations (such as Dieulafoy lesions), Mallory-Weiss tears, pancreatitis, and iatrogenic causes (2).
Endoscopy is the primary invasive technique for the diagnosis and treatment of UGI bleeding. When medical and endoscopic treatments fail, surgery or interventional embolotherapy are the treatment options. After the first report of selective arterial embolization of gastroepiploic artery for the control of acute gastric bleeding (3), improvements in interventional devices and embolic materials and wider availability of skilled interventional radiologists have increased the utility of embolization procedures in the management of UGI bleeding.
The typical candidate patient presents with the following: 1) massive bleeding (transfusion requirement of at least 4 U blood over 24 hours) or hemodynamic compromise (systolic blood pressure < 100 mm Hg and heart rate > 100 beats per minute or clinical shock), 2) endoscopy-refractory acute UGI bleeding, 3) recurrent bleeding after surgery. In general, the more hemodynamically unstable a patient is, the greater the chance of identifying the source of bleeding.
There are no absolute contraindications because angiography and embolization may be needed as lifesaving procedures. For patients with severe reactions to iodinated contrast media, alternative contrast agents such as carbon dioxide can be used. Relative contraindications include renal insufficiency, contrast allergy, and uncorrectable coagulopathy. There is increased risk of gastric or duodenal infarction after embolotherapy in patients with previous extensive UGI surgery or radiotherapy. If the rate of bleeding is massive, surgery may be preferable to angiography, since angiography may not be able to control the bleeding as quickly as surgery (4).
Screen-film arteriography can demonstrate bleeding at rates as low as 0.5 mL/min in a canine model (5), but in vitro studies suggest that digital subtraction arteriography is five to nine times more sensitive than screen-film arteriography in detecting hemorrhage (6), which seems to be equivalent to detection rates of scintigraphic images (7). The major limitation of angiography relates to the intermittent nature of bleeding, which can result in a negative study if the bleeding has temporarily stopped at the time of contrast injection.
Glucagon and Buscopan may be given before the procedure to decrease bowel motility and motion artifacts during digital subtraction angiography (8). Aortograms are usually not performed, since visualization of contrast extravasation into the GI tract requires more selective injection (4). The vessel selected first should be based on the suspicion of the likely source of bleeding according to history, clinical signs, as well as localization provided by scintigraphic images or computed tomography scans. For UGI bleeding, the celiac and superior mesenteric arteries (SMAs) are the primary targets. If there is no sign of active bleeding on injection of the main trunks, more selective injection may be needed. For duodenal or gastric fundus bleeding, the gastroduodenal artery (GDA), or left gastric arteries, respectively, should be studied (4). Obtaining images should be continued until the venous phase has cleared out to help distinguish contrast extravasation from persistent venous opacification.
The only direct angiographic sign of UGI bleeding is extravasation of contrast medium into the bowel lumen (2). Indirect signs include aneurysms/pseudoaneurysms, vessel irregularity, vessel cutoff and arteriovenous/arterioportal shunting, neovascularity, and increased vascularity from dilated arterioles (as seen in angiodysplasia). It is important to image into the venous phase.
When a bleeding source is not identified because GI bleeding is often intermittent, provocative angiography may improve the rate of positive angiographic findings. Infusion of tolazoline (vasodilators), heparin, or even thrombolytics like tissue plasminogen activator, stimulate bleeding to allow the pathology to be localized. In one study based on 16 patients with nonlocalized lower GI bleeding from previous endoscopic and angiographic studies, these infusions identified the bleeding site in 37.5% of cases without causing any hemodynamic instability (9).
The choice of embolic agent depends on a combination of the vascular anatomy, angiographic findings, the achievable catheter position, and the operator preference (Table 1). The most common embolic agents are metallic coils and polyvinyl alcohol (PVA) particles (usually 300-500 µm). Use of coils as the only embolic agent is significantly associated with early rebleeding, compared with the use of PVA or gelatin sponge with coils (10, 11). Use of N-butyl cyanoacrylate (NBCA) is advantageous for massive bleeding that requires urgent hemostasis, especially in patients with coagulopathy because of rapid embolization (12).
Types of embolization can be classified into localized, proximal, and segmental embolizations (Fig. 1). Localized embolization is possible when the bleeding point is superselected. Proximal embolization is defined when a microcatheter could not enter the bleeding point and embolization is done in its parent artery, while the bleeding point and its distal part are not embolized. Segmental embolization is when an adjacent branch artery or arteries are included to be embolized. With proximal embolization, recanalization of the bleeding point can occur due to distal back flow. With excessive segmental embolization, ischemic complications of the involved bowel can occur.
One of the useful maneuvers in localizing culprit vessels requires clips to be placed around the area of bleeding during pre-embolization endoscopy. The clips remain in position for several hours and allow for an educated guess of the location of the culprit vascular branch (13, 14). If no extravasation is seen despite the injection of contrast, then the branches terminating at the clip are superselected using microcatheter techniques and embolized. Rotational angiography can be useful to determine the relationship between the metallic clip and a culprit vessel (14).
Vasopressin infusion works by constricting the mesenteric vessels and contraction of the smooth muscle in the bowel wall, thus reducing the blood flow to the site of bleeding and inducing the formation of a stable clot at the bleeding site. Patients should be on continuous cardiac monitoring, since vasopressin can induce coronary vasoconstriction. Infusion is started at 0.1 units/min. Repeat angiography is performed after 20 minutes to ensure that bleeding has stopped and that the vessels are not overconstricted. If bleeding persists, the dose is increased to 0.2 units/min. Infusion is then increased to 0.4 units/min if bleeding persists. Excessive vasoconstriction can lead to bowel infarction. It is known to be effective in patients with microcatheter-inaccessible bleeding or diffuse bleeding (such as hemorrhagic gastritis). However, disadvantages include the rate of rebleeding after discontinuation of the infusion, cardiovascular complications, and the difficulty in maintaining a selective catheter position. In one study, the success rate of 52% for vasopressin was inferior than that of 88% for embolization in major GI bleeding (15).
The GDA has a dual supply from both the hepatic artery and SMA. Back pressure may allow continued bleeding if embolization is performed proximally. A microcatheter is advanced beyond the bleeding site and embolization is done to prevent backflow to the bleeding site. Then, the microcatheter is withdrawn, depositing more embolic agent, until the embolization takes place proximal to the bleeding site, using the so-called sandwich technique. Likewise, bleeding from the pancreaticoduodenal arcade should be treated by embolizing the arteries both proximal and distal to the lesion. GI bleeding from GDA stump is quite common after pancreaticobiliary surgery (Fig. 2). Embolization both proximal and distal to the GDA stump or stent-graft placement could be an option. Although hepatic ischemia or infarction develops in 30-40% of patients with embolization of both proximal and distal to the GDA stump (Fig. 2), patent portal vein and extensive collateral pathways, including the inferior phrenic arteries and gastric arteries, usually serve to protect the liver from ischemic insult (16). Stent-graft placement is better than embolization for preserving intrahepatic arterial flow. Postembolization 'check' angiograms from both the celiac axis and SMA sides are required to ensure that no collateral supply to the bleeding site is present.
Embolization of pseudoaneurysms needs to be mentioned. Packing the sac of the pseudoaneurysm with coils should be avoided because this rebleeding is almost inevitable (16, 17). Because the wall of the pseudoaneurysm is weak, the breakdown or rupture of the weaker pseudoaneurysm (than normal adjacent arterial wall) seems to occur after only sac packing with embolic materials. Therefore, the sandwich technique of both proximal and distal to the bleeding site should be done. In those cases with microcatheter-inaccessible bleeding point, particulate or liquid embolic agents should be utilized. In cases requiring deeper penetration, liquid embolic materials such as NBCA could be an option (Fig. 3).
Six recent representative studies of embolization for nonvariceal UGI bleeding in 299 patients showed technical success rates of 92-100% and clinical success rates of 51-94% (11, 18-22). Rebleeding rates were 9-47%, rates for surgery were 0-35%, and 30-day mortality rates were 3-27%. A wide range of clinical success and rebleeding rates, rates for surgery, as well as 30-day mortality rates seem to have originated from different etiologies and clinical severities.
Major factors associated with rebleeding include coagulopathy, longer time to angiography, massive transfusion, previous surgery, multi-organ system failure, bleeding secondary to trauma, invasive procedures, cancer bleeding (rather than non-cancer bleeding), or use of coils as the only embolic agent (11, 21-24). In one study (25), 18 of 32 patients (56%) had a diagnosis of a coagulopathy prior to arrival to the angiographic suite; the angiographic success rate in this group of patients was 100% and the clinical success rate was 83% in spite of their coagulopathy. For patients with coagulopathy, NBCA could be an excellent option because NBCA does not depend on the coagulation process (22, 25). In another study (22), coagulopathy was observed in 41% (29/71 patients), and the clinical success rate with and without coagulopathy was not significantly different.
Because of the intermittent nature of many incidents of UGI bleeding, the incidence of a normal angiogram in a patient with acute upper and lower GI bleeding was 52% (75 out of 143 patients) in one recent report (26). In the latter study, the incidence of an angiographically negative outcome was significantly higher in patients with a stable hemodynamic status, or in patients with lower GI bleeding. During follow-up, most patients (80%, 60/75) with a negative bleeding focus experienced spontaneous resolution of their condition without rebleeding. When no evidence of bleeding is found on angiography, blind or prophylactic embolization (usually with gelatin sponge and/or coils), defined as embolization without angiographic proof of extravasation, could be an alternative and is typically guided by endoscopic information regarding the location of the bleeding vessel (2, 10, 27, 28). Although a high rate of clinical success cannot be expected with such prophylactic embolization because of possible intermittent nature of the GI bleeding, especially in gastric cancer patients, a significant proportion of such patients will benefit (10, 27, 28).
Patients with angiographic extravasation show a marked decrease in mortality when embolization is successful, compared with patients requiring surgery after failed embolization (38 vs. 83%) (29). Overall, survival is strongly correlated with clinical failure. In one study, patients with a successful embolization had one-sixth of the mortality rate of those with a failed embolization regardless of their clinical condition (11%, 10 of 95 vs. 68%, 44 of 68) (24). Coagulopathy, rescue surgery after a failed attempt for embolization, underlying medical problems such as cirrhosis and malignancy, and multisystem organ failure are related with poor survival (2, 10, 24).
Access site hematoma and pseudoaneurysms, arterial dissection, contrast allergic reactions, and nephrotoxicity can occur with the same frequency as in other endovascular procedures.
Arterial embolization in the UGI tract above the ligament of Treitz is generally considered very safe because of the rich collateral supply to the stomach and duodenum. However, the risk of significant ischemia or stricture could be increased when potential collateral vessels are damaged from previous upper abdominal surgery, radiotherapy, or severe atherosclerosis, or when liquid agents such as NBCA, or very small particles advance far into the vascular bed (22, 23, 30) (Fig. 4). Lang (31) reported a 16% (9/57 patients) incidence rate of duodenal strictures following embolization. If true bowel infarction occurs, surgical resection is generally required. For more chronic ischemic complications such as bowel stricture, balloon dilation may be possible, but surgical resection should be considered if the stricture is resistant to balloon dilation.
Nontarget embolization involves embolic material inadvertently passing into a vascular bed that was not the intended target. This may result from excessive pressure when injecting flow-directed particles or from buckling of the delivery catheter out of the target vessel during coil deployment. Removal can be attempted for the embolic materials blocking a critical vessel. Suction with a catheter can be used for some particulate emboli, but errant coils need to be retrieved with snares.
Nonvariceal UGI bleeding remains an often serious clinical challenge. A multidisciplinary approach of endoscopists, surgeons, and interventional radiologists is important. With improvements in catheter-based therapy and endovascular device development, angiography and embolization procedures is considered the gold standard for acute nonvariceal UGI bleeding refractory to endoscopy. Interventional radiologists should also be familiar to several clinical and technical factors which affect the clinical outcome of embolotherapy in certain settings.
Figures and Tables
Fig. 1
Three patterns of embolization according to embolization level for non-terminal (A) and terminal (B) arteries.
In localized embolization (1), superselective embolization is done for bleeding point without embolizing other adjacent arteries. In proximal embolization (2), e.g. microcatheter-inaccessible bleeding point, only the artery proximal to the bleeding point is embolized. In segmental embolization (3), not only bleeding point but also adjacent branch artery or arteries are embolized together.

Fig. 2
63-year-old woman with pylorus-preserving pancreaticoduodenectomy for ampulla of Vater cancer.
A. Celiac angiogram shows intraluminal bleeding (arrows) from gastroduodenal artery (GDA) stump to jejunal loops. B. Celiac angiogram immediately after microcoil embolization shows complete embolization of both proximal and distal portions of GDA stump. Note minimal collateral (arrowhead) from left gastric artery. C. Axial contrast-enhanced computed tomography scan one day after embolization shows multifocal hepatic infarctions (asterisks) in liver. D. Computed tomography follow-up one month after embolization shows complete disappearance of hepatic infarctions.
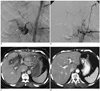
Fig. 3
54-year-old man with ERCP-induced pancreatitis.
This patient had undergone embolization for bleedings from short gastric and left interior phrenic arteries. A. Superior mesenteric artery (SMA) angiogram shows pseudoaneurysm (arrow) at distal gastroduodenal artery. B. The tip (arrowhead) of microcatheter was advanced as far as to pseudoaneurysm (arrow), however, could not reach it. C. N-butyl cyanocrylate (NBCA) embolization (1 : 3 mixture with lipiodol) was done with NBCA cast covering pseudoaneurysm. D. Completion SMA angiogram shows no visualization of pseudoaneurysm. Hematochezia was controlled.
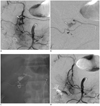
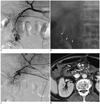
Fig. 4
65-year-old man with B-cell lymphoma presented with melena.
A. Celiac angiogram shows contrast extravasation (arrows) from small branches of gastroduodenal artery. B, C. Long segmental embolization was done with N-butyl cyanocrylate (NBCA) to prevent back flow from the superior mesenteric artery. Spot radiograph (B) and superior mesenteric artery angiogram (C) show NBCA cast (arrowheads) without further bleeding. D. A computed tomography scan 4 days later showed bowel ischemia and perforation (arrows) with peritonitis surrounding radiopaque embolic material (arrowheads).
References
1. Mirsadraee S, Tirukonda P, Nicholson A, Everett SM, McPherson SJ. Embolization for non-variceal upper gastrointestinal tract haemorrhage: a systematic review. Clin Radiol. 2011. 66:500–509.
2. Loffroy R, Rao P, Ota S, De Lin M, Kwak BK, Geschwind JF. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol. 2010. 33:1088–1100.
3. Rösch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972. 102:303–330.
4. Darcy M. Kandarpa K, Machan L, editors. Acute Gastrointestinal Arterial Bleeding. 2011. PA: Lippincott Williams & Wilkins;233–239.
5. Nusbaum M, Baum S. Raduographic demonstration of unknown sites of gastrointestinal bleeding. Surg Forum. 1963. 14:374–375.
6. Krüger K, Heindel W, Dölken W, Landwehr P, Lackner K. Angiographic detection of gastrointestinal bleeding. An experimental comparison of conventional screen-film angiography and digital subtraction angiography. Invest Radiol. 1996. 31:451–457.
7. Hastings GS. Angiographic localization and transcatheter treatment of gastrointestinal bleeding. Radiographics. 2000. 20:1160–1168.
8. Burke SJ, Golzarian J, Weldon D, Sun S. Nonvariceal upper gastrointestinal bleeding. Eur Radiol. 2007. 17:1714–1726.
9. Ryan JM, Key SM, Dumbleton SA, Smith TP. Nonlocalized lower gastrointestinal bleeding: provocative bleeding studies with intraarterial tPA, heparin, and tolazoline. J Vasc Interv Radiol. 2001. 12:1273–1277.
10. Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001. 12:195–200.
11. Loffroy R, Guiu B, D'Athis P, Mezzetta L, Gagnaire A, Jouve JL, et al. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleeding. Clin Gastroenterol Hepatol. 2009. 7:515–523.
12. Toyoda H, Nakano S, Kumada T, Takeda I, Sugiyama K, Osada T, et al. Estimation of usefulness of N-butyl-2-cyanoacrylate-lipiodol mixture in transcatheter arterial embolization for urgent control of life-threatening massive bleeding from gastric or duodenal ulcer. J Gastroenterol Hepatol. 1996. 11:252–258.
13. Eriksson LG, Sundbom M, Gustavsson S, Nyman R. Endoscopic marking with a metallic clip facilitates transcatheter arterial embolization in upper peptic ulcer bleeding. J Vasc Interv Radiol. 2006. 17:959–964.
14. Song JS, Kwak HS, Chung GH. Nonvariceal upper gastrointestinal bleeding: the usefulness of rotational angiography after endoscopic marking with a metallic clip. Korean J Radiol. 2011. 12:473–480.
15. Gomes AS, Lois JF, McCoy RD. Angiographic treatment of gastrointestinal hemorrhage: comparison of vasopressin infusion and embolization. AJR Am J Roentgenol. 1986. 146:1031–1037.
16. Gwon DI, Ko GY, Sung KB, Shin JH, Kim JH, Yoon HK. Endovascular management of extrahepatic artery hemorrhage after pancreatobiliary surgery: clinical features and outcomes of transcatheter arterial embolization and stent-graft placement. AJR Am J Roentgenol. 2011. 196:W627–W634.
17. Song HH, Won YD, Kim YJ. Transcatheter N-butyl cyanoacrylate embolization of pseudoaneurysms. J Vasc Interv Radiol. 2010. 21:1508–1511.
18. Holme JB, Nielsen DT, Funch-Jensen P, Mortensen FV. Transcatheter arterial embolization in patients with bleeding duodenal ulcer: an alternative to surgery. Acta Radiol. 2006. 47:244–247.
19. Loffroy R, Guiu B, Cercueil JP, Lepage C, Latournerie M, Hillon P, et al. Refractory bleeding from gastroduodenal ulcers: arterial embolization in high-operative-risk patients. J Clin Gastroenterol. 2008. 42:361–367.
20. Larssen L, Moger T, Bjørnbeth BA, Lygren I, Kløw NE. Transcatheter arterial embolization in the management of bleeding duodenal ulcers: a 5.5-year retrospective study of treatment and outcome. Scand J Gastroenterol. 2008. 43:217–222.
21. Poultsides GA, Kim CJ, Orlando R 3rd, Peros G, Hallisey MJ, Vignati PV. Angiographic embolization for gastroduodenal hemorrhage: safety, efficacy, and predictors of outcome. Arch Surg. 2008. 143:457–461.
22. Koo HJ, Shin JH, Yoon HK, Ko GY, Gwon DI. Transcatheter arterial embolization of gastrointestinal tract bleeding with N-Bytyl-2-Cyanoacrylate in 71 patients at a single institution. Abstract of oral presentation of The 5th Meeting of Society of Gastrointestinal Intervention.
23. Walsh RM, Anain P, Geisinger M, Vogt D, Mayes J, Grundfest-Broniatowski S, et al. Role of angiography and embolization for massive gastroduodenal hemorrhage. J Gastrointest Surg. 1999. 3:61–65. discussion 66.
24. Schenker MP, Duszak R Jr, Soulen MC, Smith KP, Baum RA, Cope C, et al. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001. 12:1263–1271.
25. Jae HJ, Chung JW, Jung AY, Lee W, Park JH. Transcatheter arterial embolization of nonvariceal upper gastrointestinal bleeding with N-butyl cyanoacrylate. Korean J Radiol. 2007. 8:48–56.
26. Kim JH, Shin JH, Yoon HK, Chae EY, Myung SJ, Ko GY, et al. Angiographically negative acute arterial upper and lower gastrointestinal bleeding: incidence, predictive factors, and clinical outcomes. Korean J Radiol. 2009. 10:384–390.
27. Morris DC, Nichols DM, Connell DG, Burhenne HJ. Embolization of the left gastric artery in the absence of angiographic extravasation. Cardiovasc Intervent Radiol. 1986. 9:195–198.
28. Lee HJ, Shin JH, Yoon HK, Ko GY, Gwon DI, Song HY, et al. Transcatheter arterial embolization in gastric cancer patients with acute bleeding. Eur Radiol. 2009. 19:960–965.
29. Dempsey DT, Burke DR, Reilly RS, McLean GK, Rosato EF. Angiography in poor-risk patients with massive nonvariceal upper gastrointestinal bleeding. Am J Surg. 1990. 159:282–286.
30. Loffroy R, Guiu B, Cercueil JP, Krausé D. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol. 2009. 7:250–263.
31. Lang EK. Transcatheter embolization in management of hemorrhage from duodenal ulcer: long-term results and complications. Radiology. 1992. 182:703–707.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download