Abstract
Objective
The aim of this study was to evaluate the frequency of postoperative biliary stricture and its risk factors in patients undergoing surgery for type I choledochal cyst.
Materials and Methods
A total of 35 patients with type I choledochal cyst underwent laparoscopic cyst excision and Roux-en-Y hepaticojejunostomy between August 2004 and August 2011. Their medical records and radiologic images (including endoscopic retrograde cholangiopancreatography, magnetic resonance cholangiopancreatography, pancreatobiliary computed tomography, or ultrasound) were retrospectively analyzed to evaluate the frequency of postoperative biliary stricture and its risk factors.
Results
Postoperative biliary stricture was found in 10 (28.6%) of 35 patients. It developed more frequently in patients with type Ia choledochal cyst (53.8%, 7 of 13 patients) than in patients with type Ic choledochal cyst (13.6%, 3 of 22 patients), which was statistically significant (p = 0.011). There were no significant associations between other factors and postoperative biliary stricture.
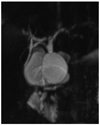
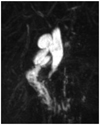
Choledochal cysts have different forms of manifestation according to Todani's classification (1). There are five types of choledochal cysts (1). The most common form is type I (2). It can be further classified into Ia, Ib (a very rare form), and Ic (1). The classification of subtypes has been of academic interest, not receiving significant clinical attention. Type Ia is formed like a saccule, into which the hepatic duct drains (Fig. 1) (1, 3). In contrast, type Ic is a fusiform dilatation of the bile duct without abrupt change of ductal caliber (Fig. 2) (1, 3). As the anatomical configuration of type Ia and Ic differ, it would be reasonable to elucidate the clinical significance of anatomic differences in perioperative management of choledochal cysts. When performing hepaticojejunostomy for type I choledochal cyst, hepaticojejunostomy anastomosis has been done occasionally to small caliber vessels, which may lead to strictures postoperatively. Therefore, it is necessary to evaluate the frequency of postoperative biliary strictures and their risk factors in patients undergoing surgery for type I choledochal cyst.
Betweme August 2004 and August 2011, 44 patients underwent laparoscopic total cyst excision and Roux-en-Y hepaticojejunostomy for choledochal cyst. According to the modified Todani's classification, the numbers of patients with type I and type IV-A were 35 (79.5%) and 9 (20.5%), respectively (1). Excluding type IV-A, a total 35 patients with choledochal cyst type I were analyzed via their medical records and radiologic images retrospectively. There were 11 (31.4%) male and 24 (68.6%) female patients. The mean age was 29.2 (2-56) years.
Preoperative radiologic studies performed were endoscopic retrograde cholangiopancreatography, magnetic resonance cholangiopancreatography (MRCP) or pancreatobiliary computed tomography (PBCT). Type I choledochal cyst can be further subclassified into types Ia and Ic according to the modified Todani's classification of choledochal cysts (1). We have analyzed the risk factors of biliary stricture of hepaticojejunostomy in type I choledochal cyst. The risk factors which we should analyze include subclassification of choledochal cyst type I, sex, age, bilirubin level, pancreatitis, symptom duration, size of cyst, presence of anomalous pancreatobiliary ductal union (APBDU), operative time, and postoperative bile leak.
Radiologic studies of MRCP, PBCT or ultrasonography (US) were performed during follow-up. The purpose of the postoperative radiologic study was to check whether biliary stricture of hepaticojejunostomy is present. The definitions of biliary stricture and relative stricture were as follows; 1) intrahepatic duct (IHD) dilatation on post operative image such as PBCT, MRCP or US, 2) serum liver function test abnormalities such as alkaline phosphatase, bilirubin or gamma glutamyl transferase elevations, 3) symptoms of cholangitis such as fever, right upper quadrant pain with serum C-reactive protein elevation and 4) recurrent IHD stone formation (4-8). The presence of biliary stricture was confirmed if any one of the above criteria was met. Statistical analysis was performed with Pearson's chi-square test, Fischer's exact test and the Mann-Whitney U-test using SPSS (ver 18.0, Chicago, IL, USA). A value of p < 0.05 was considered statistically significant.
Postoperative biliary stricture occurred in 10 patients; 7 (53.8%) patients of type Ia and 3 (13.6%) patients of type Ic. There was a significant difference in incidence of this complication between type Ia and Ic (p = 0.011) (Table 1).
In type Ia, there were four patients with IHD dilatation with cholangitis or liver function test (LFT) abnormalities, 1 patient with epigastric discomfort with LFT abnormalities, 1 patient with postprandial discomfort with LFT abnormalities, and 1 patient with LFT abnormalities without any other symptoms. Two of the above 7 patients underwent a balloon dilatation procedure and biliary stent placement procedure, respectively, and the remaining patients recovered with conservative management. In type Ic, there were 3 IHD dilatations with cholangitis. One of these patients had additional IHD stones. Among the 3, 2 patients underwent balloon dilatation and 1 patient had IHD stones removed with a choledochoscope (Table 2).
There was no correlation between biliary stricture and factors such as sex, age, laboratory data, APBDU, operation time and postoperative bile leakage (Table 1).
Type I choledochal cyst can be subclassified into types Ia, Ib, and Ic (1, 3). Among them, the clinical significance of type Ib is weak, because the incidence is so rare (2).
In the clinical setting, type Ia choledochal cyst tended to have larger diameter saccules with a draining bile duct, which was occasionally narrow. The shape of type Ia cysts resembled an upside-down funnel. The junction between the common hepatic duct and the upper part saccule may not have enough diameter for anastomosis in a type Ia cyst. Therefore, we have difficulties during hepaticojejunostomy in type Ia choledochal cyst patients compared with the fusiform type Ic. We believe that these anatomical characteristics may affect operative outcomes such as postoperative hepaticojejunostomy stricture. The overall incidence of hepaticojejunostomy strictures have been reported to be 6-40% (5, 9-11). In a study comparing types I and IV-A, Cho et al. (10) reported that definite anastomotic stricture occurred more frequently in patients with type I than in those with type IV-A. Although there have been several reports comparing postoperative outcomes between type I and IV-A (5, 9, 10, 12, 13), there were few report comparing types Ia and Ic. Our study showed that strictures occurred more frequently in type Ia than in Ic, which is a new finding. However, it is still unclear why there is a high incidence of stricture in type Ia choledochal cyst. It has been reported that the risk factors of anastomosis site stricture were old age, size of cyst and duration of preoperative symptoms (9). In our study, there was no correlation between postoperative anastomotic stricture and the above-mentioned factors. This study has shown that the probability of postoperative anastomotic stricture is predictable based on the radiologic subclassification of choledochal cysts.
The definition of biliary stricture and relative stricture are broad in our study. As abnormality of liver function test can be significantly related with bile stasis after upper abdominal surgery, Faust and Reddy (7) suggested that biliary stricture should be considered when abdnormalities of liver function test are found. Bilirubin was a predictor of malignant biliary stricture (6). Also, Tuvignon et al. (8) suggested that biliary stricture relapse may manifests as symptoms of cholangitis. Therefore, we believe that the four criteria provide a proper definition of postoperative biliary stricture.
According to previous studies, various operative techniques have been introduced to prevent strictures. A wide-caliber hepaticojejunostomy using a normal bile duct to avoid leaks and strictures is required (4, 5, 14-17). Recently, laparoscopic procedures have been applied to choledochal cyst operations. However, laparoscopic procedures have a higher level of difficulty compared with open laparotomy procedures, especially in hepaticojejunostomy procedures (18-21). Therefore, easier and useful techniques should be developed for laparoscopic choledochal cyst operations.
Through preoperative imaging studies, type Ia and Ic can be subclassified, and the difference may affect postoperative outcomes of surgery for choledochal cyst. Therefore, careful evaluation of subtypes of type I choledochal cyst is necessary to predict postoperative stricture in patients with choledochal cyst.
References
1. Todani T. Congenital choledochal dilatation: classification, clinical features, and long-term results. J Hepatobiliary Pancreat Surg. 1997. 4:276–282.
2. O'Neill JA Jr, Templeton JM Jr, Schnaufer L, Bishop HC, Ziegler MM, Ross AJ 3rd. Recent experience with choledochal cyst. Ann Surg. 1987. 205:533–540.
3. Todani T, Watanabe Y, Toki A, Morotomi Y. Classification of congenital biliary cystic disease: special reference to type Ic and IVA cysts with primary ductal stricture. J Hepatobiliary Pancreat Surg. 2003. 10:340–344.
4. Matsumoto Y, Fujii H, Yoshioka M, Sekikawa T, Wada T, Yamamoto M, et al. Biliary strictures as a cause of primary intrahepatic bile duct stones. World J Surg. 1986. 10:867–875.
5. Todani T, Watanabe Y, Urushihara N, Noda T, Morotomi Y. Biliary complications after excisional procedure for choledochal cyst. J Pediatr Surg. 1995. 30:478–481.
6. Al-Mofleh IA, Aljebreen AM, Al-Amri SM, Al-Rashed RS, Al-Faleh FZ, Al-Freihi HM, et al. Biochemical and radiological predictors of malignant biliary strictures. World J Gastroenterol. 2004. 10:1504–1507.
7. Faust TW, Reddy KR. Postoperative jaundice. Clin Liver Dis. 2004. 8:151–166.
8. Tuvignon N, Liguory C, Ponchon T, Meduri B, Fritsch J, Sahel J, et al. Long-term follow-up after biliary stent placement for postcholecystectomy bile duct strictures: a multicenter study. Endoscopy. 2011. 43:208–216.
9. Kim JH, Choi TY, Han JH, Yoo BM, Kim JH, Hong J, et al. Risk factors of postoperative anastomotic stricture after excision of choledochal cysts with hepaticojejunostomy. J Gastrointest Surg. 2008. 12:822–828.
10. Cho MJ, Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, et al. Surgical experience of 204 cases of adult choledochal cyst disease over 14 years. World J Surg. 2011. 35:1094–1102.
11. Kim JW, Moon SH, Park do H, Lee SS, Seo DW, Kim MH, et al. Course of choledochal cysts according to the type of treatment. Scand J Gastroenterol. 2010. 45:739–745.
12. Lenriot JP, Gigot JF, Ségol P, Fagniez PL, Fingerhut A, Adloff M. Bile duct cysts in adults: a multi-institutional retrospective study. French Associations for Surgical Research. Ann Surg. 1998. 228:159–166.
13. Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977. 134:263–269.
14. Tan SS, Tan NC, Ibrahim S, Tay KH. Management of adult choledochal cyst. Singapore Med J. 2007. 48:524–527.
15. Miyano T, Yamataka A, Kato Y, Segawa O, Lane G, Takamizawa S, et al. Hepaticoenterostomy after excision of choledochal cyst in children: a 30-year experience with 180 cases. J Pediatr Surg. 1996. 31:1417–1421.
16. Todani T, Watanabe Y, Toki A, Ogura K, Wang ZQ. Co-existing biliary anomalies and anatomical variants in choledochal cyst. Br J Surg. 1998. 85:760–763.
17. Stringer MD. Wide hilar hepaticojejunostomy: the optimum method of reconstruction after choledochal cyst excision. Pediatr Surg Int. 2007. 23:529–532.
18. Urushihara N, Fukuzawa H, Fukumoto K, Sugiyama A, Nagae H, Watanabe K, et al. Totally laparoscopic management of choledochal cyst: Roux-en-Y Jejunojejunostomy and wide hepaticojejunostomy with hilar ductoplasty. J Laparoendosc Adv Surg Tech A. 2011. 21:361–366.
19. Abbas HM, Yassin NA, Ammori BJ. Laparoscopic resection of type I choledochal cyst in an adult and Roux-en-Y hepaticojejunostomy: a case report and literature review. Surg Laparosc Endosc Percutan Tech. 2006. 16:439–444.
20. Tang ST, Yang Y, Wang Y, Mao YZ, Li SW, Tong QS, et al. Laparoscopic choledochal cyst excision, hepaticojejunostomy, and extracorporeal Roux-en-Y anastomosis: a technical skill and intermediate-term report in 62 cases. Surg Endosc. 2011. 25:416–422.
21. Nguyen Thanh L, Hien PD, Dung le A, Son TN. Laparoscopic repair for choledochal cyst: lessons learned from 190 cases. J Pediatr Surg. 2010. 45:540–544.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download