Abstract
Objective
To evaluate the effectiveness of ultrasound-guided radiofrequency (RF) ablation in patients with incompletely treated hepatocellular carcinoma (HCC) after transcatheter arterial chemoembolization (TACE) and to evaluate possible prognostic factors for this therapy.
Subjects and Methods
Thirty nine patients with incompletely treated single HCC (≤ 5 cm) after TACE were treated with RF ablation. All patients were evaluated for complete tumor ablation rate, local recurrence-free rate, overall survival rate, and complications. Local recurrence-free rate and overall survival rate were calculated using the Kaplan-Meier method. The possible prognostic factors of local recurrence-free rate and survival rate were analyzed using Cox proportional-hazards regression model.
Results
The complete tumor ablation rate was 92.3%. Local recurrence-free rates for 1-, 2-, 3-, and 5-years were 81.7%, 63.1%, 53.6%, and 35.7%, respectively. One-, 2-, 3-, and 5-year overall survival rates were 96.9%, 82.9%, 67.8%, and 48.4%, respectively. Among prognostic factors included in the analysis, only tumor diameter (≤ 2 cm versus > 2 cm) was statistically significant in terms of predicting local recurrence. Complications were observed in two patients, one with liver abscess and the other with portal venous thrombosis.
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies worldwide (1). Monitoring patients at high risk for HCC with imaging studies and blood tests for alpha-fetoprotein levels leads to diagnosis of many cases when there are only one or a few lesions, and the patient is still asymptomatic (2). Furthermore, several nonsurgical modalities have been developed for the treatment of unresectable HCC even with a single HCC, including cryoablation or radiofrequency (RF) ablation, percutaneous ethanol injection (PEI), and transcatheter arterial chemoembolization (TACE). Of those, RF ablation is one of the most widely performed procedures for patients with unresectable HCC because of its effectiveness and safety in the treatment of small (≤ 3 cm) and medium (≤ 5 cm) HCC, with a 3-year survival rate of 62-68%, a treatment-associated morbidity rate of 0-12%, and a treatment-related mortality rate of 0-1% (3-8).
RF ablation has recently gained attention as a more promising technique for the treatment of HCC (8-16). Many investigators have reported that RF ablation for patients with HCC provides favorable survival rates with excellent local recurrence-free rates (17).
Meanwhile, TACE has been widely used in cases of unresectable HCC during the past 20 years. But, some authors report that complete tumor necrosis after TACE is unusual (18), thus alternative treatment is considered. Even when initial remission is demonstrated after TACE, local recurred HCC is frequently observed, and subsequently repeated TACE is considered. To our knowledge, there are few studies evaluating the rate and prognostic factors for local recurrence and survival after RF ablation in patients with incompletely treated HCC who had undergone TACE. The purpose of this study was to retrospectively evaluate the effect of ultrasound-guided RF ablation in patients with incompletely treated HCC after TACE. In addition, possible prognostic factors regarding the efficacy of this therapy were also evaluated.
Between June 2002 and September 2007, 116 consecutive patients with incompletely treated HCCs after TACE were referred to the department of radiology for RF ablation. Inclusion criteria for this study were as follows: 1) a single HCC smaller than or equal to 5 cm in diameter; 2) lesions visible on ultrasonography (US), with an acceptable and safe path between the lesion and the skin as observed on the US scan; 3) no extrahepatic metastases present; 4) no imaging evidence of tumor invasion into the major portal or hepatic vein branches; 5) liver cirrhosis classified as Child-Pugh class A or B; 6) no history of encephalopathy, ascites refractory to diuretics, or variceal bleeding, and 7) no previous treatment for HCC except TACE. Consequently, 39 patients with a single HCC (33 men and 6 women; age range 48-84 years; mean age 66 years) were enrolled in this study. Tumor size ranged from 1.1 to 5.0 cm in diameter (mean, 2.4 cm).
The diagnosis of incompletely treated HCC was made when there were areas within HCC that had not undergone complete necrosis based on the follow-up CT or MR imaging performed within 1 month after TACE. These areas were considered to be present by observing incomplete accumulation of lipiodol within HCC or arterially enhancing portion within HCC on follow up CT or MR imaging (19). Of the 39 patients, 22 patients were treated with one cycle of TACE, 13 patients with 2 cycles and 4 patients with 3 cycles.
All RF ablation was performed percutaneously under real-time sonographic guidance. A 20 or 15 cm long, 17 G, Cool-tip electrode with a 2 or 3 cm long exposed metallic tip (Valleylab, Boulder, CO, USA) was used to deliver radiofrequency energy. A 200 W, 480 kHz monopolar radiofrequency generator (CC-1, Valleylab, Boulder, CO, USA) was used as the energy source. A standard grounding pad (Valleylab, Boulder, CO, USA) was placed on each of the patient's thighs.
RF ablation procedures were performed with the patient under local anesthesia in the ultrasonography suite. The most appropriate approach was determined to avoid damaging large vessels near the targeted HCC, and then a single electrode was directly inserted through the skin and positioned at the center of the HCC under US guidance using a 3.75 MHz convex probe. The tip of the electrode was then further advanced to the deepest margin of the tumor. RF energy was applied for 8-12 min in each treatment session. During the procedure, a thermocouple embedded within the electrode tip continuously measured the temperature of the tip; tissue impedance was monitored by circuitry incorporated within the generator. When the desired current could not be applied without observing an elevation in impedance suggestive of tissue boiling, the generator automatically switched to the pulsed-RF technique. At the end of treatment, the RF ablation application and cooling circuits were simultaneously interrupted, and the heated tissues were allowed to heat the electrode by diffusion until the maximum temperature was recorded. To prevent bleeding, bile leakage, and tumor seeding, the intrahepatic needle track was treated with thermocoagulation and the electrode was removed. Tumors larger than 3.5 cm needed multiple overlapping ablations (20).
One hour after the initial treatment, contrast-enhanced dynamic CT was performed. When the thickness of the ablative margin was at least 0.5 cm for ablation of index tumor, the treatment was completed. When a residual enhanced lesion was seen on dynamic CT, an additional RF ablation was performed for the residual enhanced lesion. The effectiveness of the RF ablation technique was evaluated with dynamic CT performed 1 month after the first RF ablation session was performed. When no enhancing lesion was seen on CT, the technique effectiveness was defined as complete. When residual enhancing lesion was still seen on CT, the technique effectiveness was defined as incomplete. The HCCs showing incomplete technique effectiveness were not treated with additional RF ablation but rather with TACE.
Thereafter, the patients were evaluated on scheduled follow-ups every 2-4 months with contrast-enhanced dynamic CT to detect local recurrence. Local recurrence was defined as the development of tumoral enhancement in or around the ablation zone as observed on follow-up CT. Additionally, overall survival was evaluated and defined as the interval between the first RF ablation and either death or last follow-up visit. At each follow-up visit, blood tests, including those for liver function and serum alpha-fetoprotein levels, were performed.
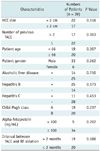
Ten possible prognostic factors were postulated and each was divided into two categories for analyses. The possible prognostic factors were tumor size (≤ 2 cm or > 2 cm maximum dimension), number of previous TACE treatments (1 or ≥ 2), patient age, patient gender, antibody against hepatitis C virus (anti-HCV) (positive or negative), hepatitis B surface antigen (HBs Ag) (positive or negative), Child-Pugh class (A or B), history of alcoholic liver disease (yes or no), level of alpha-fetoprotein (≤ 100 ng/mL or > 100 ng/mL), and interval between TACE and RF ablation (≤ 2 months or > 2 months).
The complete ablation rate and major complication rate were calculated. The local recurrence free rate and overall survival rate were evaluated using the Kaplan-Meier method. Possible prognostic factors influencing local recurrence and overall survival were analyzed using a Cox proportional hazards regression model. The level of significance (p value) was set at 0.05 for all tests. SPSS statistical analysis software (SPSS Inc.) was used.
In 36 (92.3%) of 39 patients, complete tumor ablation was depicted on dynamic CT performed 1 month after treatment (Fig. 1). The 3 patients with a single HCC showing an incomplete response were not treated with a second RF ablation treatment because of the heat sink effect from the adjacent portal vein in one patient and large diameter (5 cm and 3.8 cm, respectively) in other 2 patients (Fig. 2). Afterward, the 3 patients in whom RF ablation treatment failed underwent subsequent TACE.
No procedure-related death was observed. Complications were observed in two (5.1%) of 39 patients. Hepatic abscess, which was a major complication, occurred in one patient. The patient had developed a high fever 7 days after RF ablation and the CT findings in the ablated zone was similar to those of a hepatic abscess. Subsequently, US-guided percutaneous drainage was performed. Portal venous thrombosis was observed in the other patient which was a minor complication.
The follow-up periods ranged from 90 to 1950 days (mean 767 days). The local recurrence free rates at 1, 2, 3, and 5 years were 81.7%, 63.1%, 53.6%, and 35.7%, respectively (Fig. 3). During the follow-up period, local recurrence developed in 13 (33.3%) of 39 patients. At the time of local tumor recurrence, distant recurrence was observed in 3 (7.7%) of 39 patients. Tumor diameter was found to be a significant prognostic factor affecting local recurrence (p = 0.047) (Table 1).
TACE is a widely used therapeutic modality for unresectable HCC and has an excellent anti-tumor effect (21-23). However, the efficacy of TACE remains controversial (24) because HCC is sometimes resistant to TACE treatment. Hashimoto et al. (25) reported that TACE induced complete necrosis only in 50% of single nodular type tumors.
While treatment of TACE-resistant HCC is a major clinical objective, RF ablation has been accepted as a safe and useful technique for the local treatment of HCC. In the present study, we found that RF ablation for incompletely treated HCC after TACE produced a relatively high complete ablation rate. Our findings are consistent with previous studies. Shibata et al. (26) reported that complete ablation was achieved in 46 (96%) of 48 HCCs (≤ 4 cm) treated with RF ablation. Lencioni et al. (16) reported that complete ablation was achieved in 63 (91%) of 69 HCCs (≤ 5 cm). Moreover, the side effects and the long-term impairment of liver function associated with TACE (27, 28) further supports the use of RF ablation rather than subsequent repeated TACE treatments.
Of the three cases we considered to result in incomplete ablations, two cases were relatively large tumors that were 5 cm and 3.8 cm in diameter, respectively. It was reported that in HCCs less than 3 cm in diameter, complete ablation rate was 90%, but complete ablation rate was 71% in HCCs ranging 3-5 cm and 25% in HCCs over 5 cm in diameter (9, 12, 29). In the third case, we found the HCC was located between large branches of hepatic vessels, so it may have caused incomplete ablation because blood flow promotes heat loss, creating a so called heat sink effect.
Our study achieved comparable local recurrence-free rates and survival rates as demonstrated in previous studies of RF ablation alone for the treatment. Other studies have reported rates of local recurrence after RF ablation for HCC of 19% to 36% (30-35), which is in accordance with the results of our study. Also, numerous studies demonstrated the 5-year post-RF ablation overall survival rates ranged from 33% to 58%, supporting that RF ablation may be accepted as primary treatment for patients with HCC for whom hepatectomy or liver transplantation was not suitable (5, 8, 17, 31-33, 36).
We were concerned about possible influences of prognostic factors on local recurrence-free rates and survival rates. However, we found only HCC size was a significant prognostic factor in local recurrence-free rates. Local recurrence-free rate was higher in patients with small HCC (≤ 2 cm). Generally, RF ablation treatment is known to induce a spherical thermal lesion measuring approximately 3 cm at its greatest dimension in a single session (37). Accordingly, ablation of HCCs larger than 3 cm may require repeated sessions. Komorizono et al. reported that an HCC dimension > 2 cm was associated independently with local recurrence after a single session RF ablation, which is in accordance with the results of our study (38).
Complications after RF ablation for HCC have been reported. The rate of complications for the treatment of HCC was reported to be greater than 5% (12, 14, 17, 26). The relatively high rate of complications may be due to the prevalence of liver cirrhosis in patients treated. The complications we observed in our investigation were similar to those of studies dealing with HCC associated with liver cirrhosis.
Our study has several limitations. First, this study was retrospectively performed and was not randomized in design. Second, most HCCs were not pathological confirmed. Third, this study included a relatively small number of patients.
In conclusion, ultrasound-guided RF ablation could be an effective and safe method for treating incompletely treated HCC after TACE. The diameter of HCC was a significant prognostic factor for local recurrence.
Figures and Tables
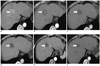 | Fig. 13 cm HCC in 66-year-old woman with no local recurrence during long term follow-up (3 years).
A. Axial arterial-phase CT scan obtained before RF ablation shows early enhancement (arrow) in sparsely lipiodol uptake HCC after TACE. B. Axial arterial-phase CT scan obtained 1 hour after RF ablation shows ablation zone (arrow) with surrounding rim of benign enhancement. C-F. Axial arterial-phase CT scans from 1-month (C), 1-year (D), 2-year (E) and 3-year (F) follow-ups show progressive involution of ablation zone without local recurrence (arrow). HCC = hepatocellular carcinoma, TACE = transcatheter arterial chemoembolization, RF = radiofrequency
|
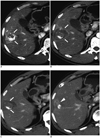 | Fig. 23.8 cm HCC in 48-year-old man with incomplete tumoral necrosis.
A. Axial arterial-phase CT scan obtained before RF ablation shows early enhancement (arrows) in defective lipiodol uptake HCC after TACE. B. Axial arterial-phase CT scan obtained 1 hour after RF ablation shows irregular perilesional enhancement (arrows). C. Axial arterial-phase CT scan obtained 1 month after RF ablation shows disappearance of perilesional enhancement (arrows). D. CT scan obtained at more superior level than C shows enhancing portion (arrowhead) at periphery of ablation zone, which was subsequently treated with TACE. HCC = hepatocellular carcinoma, TACE = transcatheter arterial chemoembolization, RF = radiofrequency
|
 | Fig. 3
Graph illustrates local recurrence free rate. 1-, 2-, 3-, and 5-year local recurrence free rates were 81.7%, 63.1%, 53.6%, and 35.7%, respectively. |
 | Fig. 4
Graph illustrates overall survival rate. 1-, 2-, 3-, and 5-year overall survival rates were 96.9%, 82.9%, 67.8%, and 48.4%, respectively. |
References
1. El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002. 35:S72–S78.
2. Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. 2006. 16:661–669.
3. Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002. 235:466–486.
4. Cammà C, Di Marco V, Orlando A, Sandonato L, Casaril A, Parisi P, et al. Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): a prospective study. J Hepatol. 2005. 42:535–540.
5. Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005. 103:1201–1209.
6. Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000. 7:593–600.
7. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006. 243:321–328.
8. Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001. 11:914–921.
9. Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998. 170:1015–1022.
10. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–661.
11. Dodd GD 3rd, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000. 20:9–27.
12. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.
13. Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000. 217:633–646.
14. McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001. 176:3–16.
15. Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol. 2001. 12:1135–1148.
16. Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003. 228:235–240.
17. Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005. 234:961–967.
18. Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, et al. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology. 1987. 163:345–351.
19. Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2007. 18:1469–1478.
20. Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology. 2004. 232:260–271.
21. Lau WY, Yu SC, Lai EC, Leung TW. Transarterial chemoembolization for hepatocellular carcinoma. J Am Coll Surg. 2006. 202:155–168.
22. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002. 35:1164–1171.
23. Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983. 148:397–401.
24. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003. 362:1907–1917.
25. Hashimoto T, Nakamura H, Hori S, Tomoda K, Nakanishi K, Murakami T, et al. Hepatocellular carcinoma: efficacy of transcatheter oily chemoembolization in relation to macroscopic and microscopic patterns of tumor growth among 100 patients with partial hepatectomy. Cardiovasc Intervent Radiol. 1995. 18:82–86.
26. Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002. 223:331–337.
27. Yamashita Y, Torashima M, Oguni T, Yamamoto A, Harada M, Miyazaki T, et al. Liver parenchymal changes after transcatheter arterial embolization therapy for hepatoma: CT evaluation. Abdom Imaging. 1993. 18:352–356.
28. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995. 332:1256–1261.
29. Francica G, Marone G. Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a 'cooled-tip needle'. A preliminary clinical experience. Eur J Ultrasound. 1999. 9:145–153.
30. Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009. 249:20–25.
31. Raut CP, Izzo F, Marra P, Ellis LM, Vauthey JN, Cremona F, et al. Significant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol. 2005. 12:616–628.
32. Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005. 29:1364–1373.
33. Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007. 17:684–692.
34. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005. 54:1151–1156.
35. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004. 127:1714–1723.
36. Cabassa P, Donato F, Simeone F, Grazioli L, Romanini L. Radiofrequency ablation of hepatocellular carcinoma: long-term experience with expandable needle electrodes. AJR Am J Roentgenol. 2006. 186:S316–S321.
37. de Baere T, Denys A, Wood BJ, Lassau N, Kardache M, Vilgrain V, et al. Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable systems. AJR Am J Roentgenol. 2001. 176:187–192.
38. Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003. 97:1253–1262.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download