Abstract
Immunoglobulin G4 (IgG4)-related sclerosing disease is a systemic disease characterized by extensive IgG4-positive plasma cells and T-lymphocyte infiltration in various organs. We described the imaging findings of an IgG4-related inflammatory pseudotumor in the urethra. The urethral mass showed isoattenuation on unenhanced CT images, delayed enhancement on enhanced CT images, iso- to slight hyper-intensity on T1 and T2 weighted magnetic resonance images, diffusion restriction on diffusion weighted images, and heterogeneously low echogeneity on ultrasonography.
Immunoglobulin G4 (IgG4)-related sclerosing disease is a systemic disease characterized by extensive IgG4-positive plasma cells and T-lymphocyte infiltration of various organs (1). Aside from autoimmune pancreatitis, it is the most common expressed form of IgG4-related sclerosing disease. Numerous other manifestations of the disease has been noted: sclerosing cholangitis, sclerosing cholecystitis, sclerosing sialadenitis, retroperitoneal fibrosis, tubulointerstitial pneumonia, prostatitis, hypophysitis (1-3). In addition, it has been reported that the IgG4-related sclerosing disease could also be represented as inflammatory pseudotumor (IPT) in various organs (1, 4, 5). To our knowledge, however, involvement of the urethra by the IgG4-related sclerosing disease has not been reported in the previous literature. In this report, we present a case of IgG4-related IPT in the urethra.
A 72-year-old woman presented with dysuria, which continued for a week. About 17 years before the presentation, the patient had a past medical history of an eyelid mass, which was clinically diagnosed as IPT and was relieved by steroid therapy. About 15 years later, she also underwent a computed tomography (CT) scan for the acute abdominal pain, and it revealed diffuse swelling of the pancreas. By a percutaneous cutting needle biopsy of the pancreas, the lesion was pathologically diagnosed as IgG4-related autoimmune pancreatitis, and the patient's symptoms were dramatically relieved by the steroid therapy.
To evaluate the patient's dysuria at this visit, a urologist performed physical examinations and laboratory studies, which yielded no positive findings suggestive of an infection or a malignancy, except for hematuria of 30 to 49 red blood cells per high power field (HPF) on a random urine analysis. On a subsequent cystoscopy, the urinary bladder was free, but a firm mass was suspected in the posterior wall of the urethra. Thus, CT and magnetic resonance (MR) imaging were performed for further characterization of the urethral mass. Two phase (unenhanced, enhanced) CT images were acquired with an 8-channel multi-detector raw CT (LightSpeed Ultra; GE Medical Systems, Milwaukee, WI, USA). In addition, MR images were acquired with a 3.0 Tesla MR scanner (Magnetom Trio Tim; Siemens Medical Solutions, Erlangen, Germany). On unenhanced CT images, the urethral mass demonstrated similar attenuation compared to the adjacent muscles. On subsequent contrast enhanced images, the mass showed a mild degree of delayed rim-enhancement (Fig. 1A).
Routine pelvic MR images also revealed a well-defined mass in the urethra with isointensity to slight hyperintensity on both T1 and T2 weighted images (repetition time [TR]/echo time [TE], 790/14, 4800/95, respectively) (Fig. 1B, C, respectively). On diffusion weighted images with a b value of 1000 s/mm2, obtained by echo-planar trace sequence (TR/TE, 4800/79; section thickness, 5 mm), the mass showed hyperintensity suggesting diffusion restriction (Fig. 1D). Concordantly, the mass showed a lower apparent diffusion coefficient value than that of the adjacent muscles (0.65 × 10-3 mm2/s, 1.39 × 10-3 mm2/s, respectively) (Fig. 1E). On gadolinium-enhanced fat-saturation T1-weighted images (TR/TE, 2.9/1.2; section thickness, 2.4 mm), the mass demonstrated rim-enhancement in the arterial phase and homogeneous enhancement in the 5-minute delayed phase (Fig. 1F, G, respectively).
To rule out a malignancy such as urethral carcinoma, the patient underwent a core needle biopsy of the urethral lesion under transvaginal ultrasonography (US) guidance. The mass was found to be encased in the urethra and showed heterogeneously low echogeneity (Fig. 1H).
The pathologic specimen showed linear spindle cell proliferation and inflammatory cell infiltration in lymphocytes and eosinophils (Fig. 1I). On immunohistochemical staining, both smooth muscle actin and IgG4 (more than 30/HPF) were positive (Fig. 1J, K, respectively). However, anaplastic lymphoma kinase was negative. All pathologic results were compatible with IgG4-related sclerosing disease, which was presented as IPT (IgG4-related IPT). Following steroid therapy for a few months, the patient's symptoms were relieved and the size of the mass was markedly decreased on follow-up MR images (Fig. 1L).
Immunoglobulin G4-related sclerosing disease is an emerging disease entity that can involve the pancreas, bile duct, gallbladder, salivary gland, retroperitoneum, kidney, prostate, and so on. It is characterized by extensive IgG4-positive plasma cells and T-lymphocyte infiltration of various organs associated with tissue fibrosis and obstructive phlebitis (1). On the other hand, IPT is a rare benign condition characterized by abundant spindle cells mixed with variable amounts of extracellular collagen, lymphocytes, and plasma cells (6). Therefore, IPT can either be a manifestation of IgG4-related sclerosing disease or a distinct simple IPT, according to the amount of IgG4-positive plasma cells.
Pathophysiologically, the role of IgG4-positive cell is still unclear in IPT (7, 8). Nonetheless, identifying the IPT and clarifying its relationship with IgG4-related sclerosing disease may be critical, because the IPT is a well-known mimicker of malignant tumors and it may show a different pathophysiology according to the presence of IgG4-positive plasma cells (8, 9). In particular, Yamamoto et al. (8) emphasized the evaluation of IgG4-positive plasma cells and the presence of obstructive phlebitis as markers for the differential diagnosis between IgG4-related IPT and an inflammatory myofibroblastic tumor (IMT). As the IMT is regarded as a neoplastic counterpart to the IPT, the evaluation of IgG4-positive plasma cells is crucial to determining an optimal treatment plan.
Unfortunately, identifying IgG4-related IPT, non IgG4-related IPT, and IMT is still an unexplored field from a radiologic point of view. Park et al. (6) studied the imaging findings of IPTs in the genitourinary tract without evaluating the association with IgG4-related sclerosing disease. Moreover, they reported that genitourinary IPT can be seen in variable patterns on US and CT, which may be attributed to varying degrees of fibrosis and inflammation (6). They also described the disease usually demonstrates delayed homogeneous enhancement, hypointensity on T2 weighted image, and diffusion restriction on MR imaging (6). These features can be regarded as projections of fibrotic change in the developing IPT (5, 6). However, the various degrees of fibrosis and the inflammatory process can result in a broad range of MR imaging manifestations (9). In our report, we were able to obtain CT, MR and US images of the IgG4-related sclerosing disease manifested as a urethral IPT. CT and US features of our case were consistent with known imaging findings of IPT (6, 9, 10). On MR imaging of our case, delayed enhancement and diffusion restriction were compatible with previous reports of the IPT. Although the presented case revealed isointense to slightly hyperintense T2 signal intensity, which is not typical of the fibrosis, this may be possibly understood as a result of the mixed inflammatory and fibrotic stages.
Meanwhile, a core needle biopsy under transvaginal US guidance was very useful in the diagnosis of our case. As a transvaginal US-guided biopsy is known as a safe and well-established method to obtain urethral and periurethral tissues (11), we believe this method should be considered to diagnose IgG4-related sclerosing disease involving the urethra.
Although the IPT as a manifestation of the IgG4-related sclerosing disease has been reported in various organs (1, 4, 5), most of the reports have provided limited information about imaging findings of the disease. Most of all, to the best of our knowledge, no report handles urethral involvement of the IgG4-related sclerosing disease. We described the imaging features of urethral involvement of the IgG4-related IPT, which was developed metachronously after other organ involvement including the eyelid and the pancreas. In patients with a past history or suspicion of IgG4-related sclerosing disease, urethral mass should be carefully considered as a potential manifestation of the IgG4-related sclerosing disease. Furthermore, acknowledging that the IgG4-related sclerosing disease may present as a urethral mass is essential to avoid unnecessary surgery or anti-cancer treatment. In some cases, a needle biopsy under transvaginal US guidance can be helpful to diagnose urethral involvement of IgG4-related sclerosing disease.
Figures and Tables
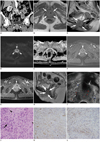 | Fig. 1CT, MR, US, and histologic findings in 72-year-old woman with IgG4-related inflammatory pseudotumor in urethra.
A. Coronal reformatted CT image reveals a rim-enhancing soft tissue around urethra (arrows). B. Axial T1-weighted MR image (TR/TE, 790/14) reveals isointense soft tissue (arrows) around urethra. C. Sagittal T2-weighted MR image (TR/TE, 4800/95) reveals iso- to slightly hyperintense mass (arrows) around urethra. D. Axial diffusion weighted MR image (TR/TE, 4800/79) demonstrates intense high signal intensity in urethral mass (arrowheads). E. Axial apparent diffusion coefficient (ADC) map demonstrates concordant low ADC values of urethral mass (arrowheads). F. On gdolinium-enhanced fat-saturation T1-weighted MR images (TR/TE, 2.9/1.2), mass (arrowheads) shows rim-enhncement in arterial phase. G. On 5-minute delayed fat-saturation T1-weighted MR image, mass (arrowheads) shows diffuse enhancement. Note that central portion of mass is enhanced in delayed phase, in comparison with arterial phase image. H. After steroid therapy for three months, sagittal T2-weighted MR image (TR/TE, 4550/107) reveals marked interval decrease in size of urethral mass (arrows). I. Transvaginal ultrasonography image in 72-year-old woman with IgG4-related inflammatory pseudotumor in urethra. transvaginal ultrasonography scan in color Doppler mode reveals heterogeneously low echoic mass (arrows) encasing urethra. Note that vascularity in urethral mass is poor. J. Microscopic exam shows linear spindle cell (arrowheads) proliferation and lymphocyte (arrow) infiltration (Hematoxylin & Eosin, × 200). K. Immunohistochemical staining reveals positivity (brown color) for marker of proliferated spindle cells and smooth muscle actin, suggesting that specimen is compatible with IPT. L. Immunohistochemical staining for IgG4 demonstrates positivity (brown color) in some inflammatory cells and spindle cells, suggesting that specimen is associated with IgG4-related sclerosing disease. IgG4 = immunoglobulin G4, IPT = inflammatory pseudotumor.
|
References
1. Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008. 14:3948–3955.
2. Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006. 6:132–137.
3. Leporati P, Landek-Salgado MA, Lupi I, Chiovato L, Caturegli P. IgG4-related hypophysitis: a new addition to the hypophysitis spectrum. J Clin Endocrinol Metab. 2011. 96:1971–1980.
4. Higashiyama T, Nishida Y, Ugi S, Ishida M, Nishio Y, Ohji M. A case of extraocular muscle swelling due to IgG4-related sclerosing disease. Jpn J Ophthalmol. 2011. 55:315–317.
5. Katsura M, Morita A, Horiuchi H, Ohtomo K, Machida T. IgG4-related inflammatory pseudotumor of the trigeminal nerve: another component of IgG4-related sclerosing disease? AJNR Am J Neuroradiol. 2011. 32:E150–E152.
6. Park SB, Cho KS, Kim JK, Lee JH, Jeong AK, Kwon WJ, et al. Inflammatory pseudotumor (myoblastic tumor) of the genitourinary tract. AJR Am J Roentgenol. 2008. 191:1255–1262.
7. Zen Y, Fujii T, Sato Y, Masuda S, Nakanuma Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol. 2007. 20:884–894.
8. Yamamoto H, Yamaguchi H, Aishima S, Oda Y, Kohashi K, Oshiro Y, et al. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. Am J Surg Pathol. 2009. 33:1330–1340.
9. Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003. 23:719–729.
10. Harr DL, Quencer RM, Abrams GW. Computed tomography and ultrasound in the evaluation of orbital infection and pseudotumor. Radiology. 1982. 142:395–401.
11. Andrich DE, Rickards D, Landon DN, Fowler CJ, Mundy AR. Structural assessment of the urethral sphincter in women with urinary retention. J Urol. 2005. 173:1246–1251.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download