Abstract
Objective
Neuroendocrine cervical carcinoma is a rare subtype of cervical cancer. These tumors exhibit an aggressive behavior with early regional lymph node and distant metastases. The purpose of our study was to describe five cases of neuroendocrine cervical-vaginal carcinoma and to discuss the potential of the 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan for the detection of this rare malignancy.
Materials and Methods
Five cases of cervical-vaginal neuroendocrine tumor were retrospectively collected, during a two year (from September 2009 to August 2011) period in our hospital. The clinical staging distributions were International Federation of Gynecology and Obstetrics (FIGO) stage IB2 (1 of 5), stage IIA (3 of 5) and stage IVA (1 of 5).
Results
Two cases (cases 1 and 4) were restaged after 18F-FDG PET/CT scan in the initial staging process. Post-treatment 18F-FDG PET/CT scans, in three patients, revealed positive findings for tumor recurrence or lymph node metastases. Two patients (cases 2 and 3) died of tumor within two years.
Conclusion
18F-FDG PET/CT scan is a useful tool in cervical-vaginal neuroendocrine tumor. In its initial staging, the 18F-FDG PET/CT scan may help assess the possible nodal involvement or early hematogeneous spreading. We can also use the 18F-FDG PET/CT to detect local recurrence and to evaluate the treatment response after clinical manipulation.
Neuroendocrine tumors arise from cells of the endocrine and nervous systems. The incidence has been estimated to be 2.5-5 per 100000 people per year with a prevalence of 35 per 100000 (1). Neuroendocrine cervical carcinoma is a rare subtype of cervical cancer with a high proliferation rate and a marked propensity for regional lymph node metastases and distant metastases, which is similar to its counterpart in the lung and other anatomic locations. Growing evidence indicates that 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan is feasible for assessing the pre-treatment staging, detecting lymph node metastases, and evaluating the treatment response in squamous cervical carcinoma. To the best of our knowledge, there are no 18F-FDG PET/CT studies with cervical-vaginal neuroendocrine tumor. Here, we describe five cases of neuroendocrine cervical-vaginal carcinoma, and discuss the potential of the 18F-FDG PET/CT scans for the detection of this rare malignancy.
From September 2009 to August 2011, five patients were diagnosed with neuroendocrine tumor in our hospital (four subjects had a tumor in the uterine cervix and one subject had a tumor in the vagina). The diagnosis of neuroendocrine carcinoma was made, according to the recently proposed classification of neuroendocrine tumors (2). All of the patients underwent 18F-FDG PET/CT scans for the initial staging procedure and three subjects received post-treatment 18F-FDG PET/CT scans for detecting possible tumor recurrences or distal metastases. This study was approved by the Ethics Committee of our Hospital (DMR-99-IRB-010).
The patients were asked to fast for at least 4 hours before scanning. Each of them was intravenously injected with 370 megabecquerel (MBq) of 18F-FDG and rested on a supine position in a quiet, dimly lit room. Imaging was performed with a PET/CT scanner (Discovery STE, General Electric Healthcare, Milwaukee, WI, USA). Scanning began approximately 40 minutes after the injection of 18F-FDG. When patients were positioned in the scanner, a molded headrest and a head-restraining Velcro band were applied to firmly secure their heads, in order to reduce motion artifact.
We used four-phase 18F-FDG PET/CT imaging protocol to improve the lesion detectability in patients with pelvic carcinoma. This protocol included the following: 1) saline infusion was given before the tracer injection. Whole-body 18F-FDG PET/CT scan was acquired, approximately 40 minutes after the intravenous injection of 370 MBq of 18F-FDG; 2) post-void delayed abdominal and pelvic 18F-FDG PET/CT images were obtained, approximately 70 minutes after 18F-FDG injection; 3) after voiding of the urinary bladder again, the patients were injected with 20 mg of furosemide intravenously. Focal supine abdominal and pelvic diuretic 18F-FDG PET/CT images were obtained, approximately 90 minutes after 18F-FDG injection; 4) additional focal imaging of the abdomen and pelvis was performed, approximately 110 minutes after 18F-FDG injection, with the patients in the prone position.
The PET/CT examinations started with the acquisition of a topogram of CT that is used to define the axial examination range of the PET/CT study. After the definition of the axial imaging range, a spiral non-contrast-enhanced low-radiation-dose CT scan (0.8-second rotation time, 120 kVp, variable mA with AutomA technique, 3.75-millimeter slice thickness and 1.75 : 1 pitch) were acquired, first, for anatomical references and attenuation correction. Then, the PET emission images were acquired after CT scans at 2 minutes per field of view (FOV) in the 3-dimensional acquisition mode with eleven-slice overlap at the borders of the FOV. The CT images were reconstructed onto a 512 × 512 matrix, and then converted to a 128 × 128 matrix, 511-keV-equivalent attenuation factors for the attenuation correction of the corresponding PET emission images. Then, the PET images were reconstructed to the same thickness of CT images, 3.27 millimeter transaxial slice thickness for further interpretation. A semiquantitative parameter, standardized uptake value (SUV), was defined as the "tracer activity in the target region per unit mass, divided by the amount of injected radioactivity, per unit body mass". The maximum standardized uptake value (SUVmax) was calculated from each region of interest with increased 18F-FDG radioactivity.
A 46-year-old female of gravida 3 and para 3 visited our emergency department with complaints of massive vaginal bleeding and abdominal fullness in July 2011. The pelvic examination revealed an exogenous mass lesion over the upper third of the vagina with malodorous discharge. Colposcopic biopsy of the cervical lesion revealed a poorly differentiated, large-cell neuroendocrine cervical carcinoma. An 18F-FDG PET/CT scan was performed, subsequently, for the demonstration of possible metastases and shown in Figure 1. The tumor was clinically staged as International Federation of Gynecology and Obstetrics (FIGO) stage IVA. The patient is currently receiving palliative radiotherapy.
A 28-year-old female suffered from malodorous vaginal spotting for one month. Pelvic examination found an area of easy bleeding over the uterine cervix, without parametrial extension, 4 cm in its largest dimension (FIGO stage IB2). The colposcopic biopsy showed neuroendocrine cervical carcinoma. A pre-treatment primary lesion was detected on the 18F-FDG PET/CT scan, as shown in Figure 2. The patient received CCRT and brachytherapy after laparoscopic bilateral ovarian transposition. Four months later, the progression of disease was evidenced by multiple lymph node and bone metastases. She was admitted for salvage chemotherapy, and was given cisplatin (500 mg/m2) and 5-FU (1000 mg/m2). Disease progression was again documented. She expired 24 months after the initial diagnosis.
A 66-year-old postmenopausal woman, with a diagnosis of stage IIA large-cell neuroendocrine cervical carcinoma was treated with radical hysterectomy, concurrent chemotherapy and radiation. Ten months after she completed her treatment, general weakness and abnormal laboratory data were noted. 18F-FDG PET/CT scan, as shown in Figure 3 was arranged for detecting possible metastases, and it revealed multiple 18F-FDG-avid lesions in the mediastinum, liver, and abdominal lymphatic chain. The patient expired from disseminated intravascular coagulopathy 21 months after diagnosis.
A 46-year-old woman was presented to our gynecology department with complaints of intermittent vaginal spotting and a malodorous vaginal discharge for 2 months. Pelvic examination, which was done under anesthesia on Dec. 3rd, 2010, showed a bulky tumor of about 4.0 cm in diameter. The pathological finding was small-cell neuroendocrine cervical carcinoma. Computed tomography scan revealed enlarged lymph nodes in the bilateral internal iliac and the left common iliac regions. The patient was treated in the outpatient setting with daily pelvic radiation therapy. However, she developed oliguria and was found to have acute renal insufficiency related to obstructive nephropathy. Bilateral double-J stents were placed to ensure the patency of the ureters. The 18F-FDG PET/CT scan of this patient is shown in Figure 4. In addition to the primary tumor seen in the pelvis, the enlarged lymph nodes shown on a CT scan were hypermetabolic. Incidentally, a hypermetabolic lesion was also noted in the left breast. The biopsy of this lesion showed metastastic neuroendocrine carcinoma. The patient is currently receiving palliative chemotherapy consisting of cisplatin (50 mg/m2), doxirubicin (50 mg/m2) and etoposide (75 mg/m2).
A 42-year-old female presented with a right vaginal septum of about 2.3 × 2.0 × 0.5 cm in size, around the middle to lower third of the vagina on the annual exam. According to her statement, she suffered from painful sensation during sexual intercourse and post-coital bleeding for nearly 2 years. Radical resection was done on December 7th, 2009. The first 18F-FDG PET/CT scan is shown in Figure 5. The final diagnosis was stage IIA large-cell neuroendocrine carcinoma. The treatment plan for this patient, which consisted of six courses of chemotherapy with concurrent radiation and four courses of brachytherapy, was completed on March 4th, 2010. However, mildly elevated carcinoembryonic antigen, 19.13 ng/dL, was noted six months later. She underwent a second 18F-FDG PET/CT scan, which showed an 18F-FDG-avid lesion in the right side of the uterine cervical region. Ongoing salvage chemotherapy was subsequently arranged in another hospital.
The clinical characteristics, treatment modalities and outcome data of the five patients with neuroendocrine cervical-vaginal carcinoma are listed in Table 1. The histopathological findings of case 2 are shown in Figure 6. Five cases of cervical neuroendocrine carcinoma were retrospectively collected within two years (from September 2009 to August 2011) at our hospital. The mean age of these patients at initial diagnosis was 44.8 years (range, 28-62 years). The clinical staging distributions were FIGO stage IB2 (1/5, 25%), stage IIA (3/5, 75%) and stage IVA (1/5, 25%). Only one patient underwent radical hysterectomy (1/5, case 3), followed by postoperative concurrent chemotherapy and radiation therapy. Two patients (2/5, cases 2 and 5) received concurrent chemotherapy and radiation therapy, one patient (1/5, case 4) received palliative chemotherapy and one patient (1/5, case 1) received palliative radiotherapy. Two patients (cases 2 and 3) died of tumor within two years.
Four primary tumors were detected by 18F-FDG PET/CT scan. The 18F-FDG PET/CT scan was performed after radical hysterectomy in case 3; therefore, no primary lesion was detected. The SUVmax of these tumors were as follows: 6.08 (Fig. 1), 9.66 (Fig. 2), 5.62 (Fig. 4), 2.21 (Fig. 5), respectively. The mean SUVmax of primary lesions was 5.89 in the early images, and 8.68 in delayed images. Distant metastases were presented in the pretreatment 18F-FDG PET/CT scan in case 1 and 4. In case 1, the highest SUVmax (11.46) was noted in the right sacroiliac joint in the 3rd phase. In case 4, the FDG-avid lesion with a highest SUVmax (5.08) in the 3rd phase was noted in the right iliac lymph nodes. The restaging 18F-FDG PET/CT in case 2 and 3 revealed previous unknown distant metastatic disease. The highest SUVmax in case 2 and 3 were 12.34 and 8.93, respectively. The mean SUVmax of the metastatic lesions was 9.45 in the 3rd phase.
Carcinoma of the uterine cervix is a common gynecologic malignancy with squamous cell histology, accounting for the majority of the cases. Rare cervical tumors include lymphoma, neuroendocrine, and signet cell tumors. Most cases of the uterine cervical carcinoma can be detected at an early stage of the disease by a Pap smear test. Staging and treatment in uterine cervical carcinoma greatly depends on the FIGO staging system. Early-stage squamous cell carcinoma of the uterine cervix has good prognosis and outcome. However, cervical-vaginal neuroendocrine tumors show evidence of distal metastases in the early stage disease (3-5). These tumors exhibit aggressive behavior, with early hematogenous and lymphatic metastases. In a recent analysis of the data from the Surveillance Epidemiology and End Results Program of the U.S. National Cancer Institute, it was stated that the neuroendocrine tumors of the uterine cervix are extremely aggressive, and that the survival is poor regardless of the stage at diagnosis, when compared with squamous cell types (6). A retrospective study, which enrolled 31 patients in northern Taiwan, demonstrated that the 2-year and 5-year survival rates were 54.8% and 31.5%, respectively, for all types of cervical neuroendocrine tumor (7). This phenomenon, including poor prognosis and metastases in the early stages, was also observed in our patients.
The staging methods of the FIGO system depend on conventional procedures, including plain chest radiography, barium enema, intravenous urography, lymphangiography, cystoscopy, proctoscopy, and physical examination, under general anesthesia. However, the FIGO system is inherently inaccurate in the advanced stage disease, and does not address nodal involvement. Therefore, the expected roles of preoperative imaging are evaluation of invasion to the adjacent organs and assessment of the nodal involvement and distant metastases.
Some recent studies reported that primary uterine cervical cancers have an avid uptake of 18F-FDG with the SUVmax, ranging from 4.0 to 33 (8-10). In our study, the SUVmax of the primary cervical-vaginal neuroendocrine tumor ranged between 2.2 and 9.6. Apparently, we cannot differentiate the primary cervical-vaginal neuroendocrine tumors from primary uterine cervical cancers empirically based on SUVmax. However, the metastatic patterns of these two types of tumors may have different appearances. The carcinoma of the uterine cervix is believed to typically advance with local growth and lymphatic spread initially, and by blood-borne metastases in the late stages. Dissemination generally follows an orderly sequence (11). Cervical cancer can direct an invasion to the vagina, paracervical and paramatrial areas. Tumor emboli spread orderly to peri-cervical, obturator, presacralthe hypogastric, external iliac, para-aortic, and then supraclavicular lymph nodes (12). In the late stages, hematogeneous dissemination could happen, and mostly commonly, to the lung, liver, and bone. On the contrary, the dissemination of cervical-vaginal neuroendocrine tumor seems not develop in an orderly manner. The neuroendocrine tumor can be associated with hematogeneous spread even when no evidence of lymphatic spreading of the tumor is noted. In our study, there are multiple bone metastases without lymph nodes involvement in case 1. In case 4, metastatic lesion in the left breast was confirmed without the lung, brain or bone involvement.
There are few articles concerning MRI findings in cervical-vaginal neuroendocrine tumor (13, 14). Yan et al. demonstrated preoperative MRI findings in seven patients with small cell neuroendocrine cervical carcinoma. In their study, 71% of patients had parametrial invasion and 86% of patients had lymphadenopathy even with small size of primary tumor. These findings may confirm the needs of further treatment after surgical manipulation. To the best of our knowledge, no study has been made to compare the diagnostic accuracies of cervical-vaginal neuroendocrine tumor, between 18F-FDG PET scan and magnetic resonance (MR) images. However, for the common types of squamous cervical cancer, a prospective study investigated the efficacy of 18F-FDG PET scan in comparison with MR images of patients, with newly diagnosed (35%) or recurrent (65%) cervical cancer (15), which showed 18F-FDG PET scan with significant superiority to that of the MRI-CT in identifying metastatic lesions, although, the diagnostic accuracy was similar for local tumors. The pretreatment 18F-FDG PET/CT scan, which can reveal abnormal 18F-FDG uptake, consistent with nodal disease, is a robust predictor of disease recurrence and may alter the therapeutic management (16). The advantage of the whole body survey in an 18F-FDG PET/CT scan may be more important in the staging of cervical-vaginal neuroendocrine tumor, since hematogenous spread can occur early in this type of tumor. Therefore, we considered that 18F-FDG PET/CT scans may be more appropriated than the local MR images for detecting the possible distant metastases, despite no evidence of local lymph node involvement. In our study, two cases were restaged (cases 1 and 4, shown in Table 1) after 18F-FDG PET/CT scan. In case 4, the initial FIGO stage was IIA. However, several 18F-FDG-avid areas in the bilateral iliac, the retroperitoneal regions, and the left breast were demonstrated in the 18F-FDG PET/CT scan, which altered the therapeutic management. The patient received palliative chemotherapy for systemic metastases, and is still alive (as of November 2011, 11 months after initial treatment). Multiple modalities, such as radical hysterectomy and node dissection, adjuvant chemotherapy, chemoradiation and brachytherapy for neuroendocine tumors, have recently been shown to improve local control and survival rates. However, the optimal treatment has not yet been completely clarified.
Approximately 30% of uterine cervical cancers eventually relapse, after the initial treatment (17). The recurrence rate in cervical-vaginal neuroendocrine tumor is even higher (18). However, the follow-up surveillance is not standardized. Follow-up protocols, including physical examination, evaluation of the serum tumor markers, ultrasonography, CT and/or MRI, have certain limitations. 18F-FDG PET scan or 18F-FDG PET/CT scan is a valuable tool in the case of suspected recurrence of cervical cancer. Cumulative data on 431 patients showed that 18F-FDG PET scan had the mean sensitivity of 94.7%, the mean specificity of 83.7% (60-100%), and the mean diagnostic accuracy of 87.9% (70-97.2%) for the detection of recurrences in the uterine cervical cancers (19). A retrospective study enrolled 40 patients, who were suspected of having cervical cancer recurrence (20). The 18F-FDG PET/CT scans in these patients (squamous or adenosquamous type on histology examination) provided higher sensitivity and accuracy rates than that of the conventional imaging (p < 10-3), with a sensitivity of 94% for PET/CT scan, compared to 42.5% for the conventional imaging. Three patients (cases 2, 3, and 5) were suspected of having recurrence in our study, and they underwent a second 18F-FDG PET/CT scan. Despite completion of the treatment (both chemotherapy and radiotherapy), rapid recurrence and metastases were found. In cases 2 and 3, lymph node metastases in the pelvis, para-aortic lymphatic chain and mediastinum were demonstrated in the second 18F-FDG PET/CT scan. Two patients died from the disease within two years (21 months and 24 months), despite the ongoing treatment.
Stage and treatment in cervical-vaginal neuroendocrine tumor remain to be determined because of the rarity of this type of cancer. Here, we described five cases of cervical-vaginal neuroendocrine tumor with early lymph node metastases and poor prognosis, despite multiple modalities of treatment planning. 18F-FDG PET/CT scan is a useful tool in cervical-vaginal neuroendocrine tumor. In initial staging, the 18F-FDG PET/CT scan may assess the possible nodal involvement or early hematogenous spreading, which altered the FIGO stage. We can also use the 18F-FDG PET/CT scan to detect local recurrence and to evaluate the treatment response after clinical manipulations. The limitations of our study are small case numbers and short-term follow-up. Further evaluation, which includes larger case numbers, is necessary.
Figures and Tables
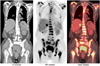 | Fig. 1
2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography images showed multiple hypermetabolic areas in skeleton (including axial bones and long bones of four limbs), retroperitoneal lymph nodes, liver, lung, uterine cervix, and posterior wall of urinary bladder. Renal cortex, hepatic parenchyma, and soft tissue uptake were faintly visualized. PET = positron emission tomography |
 | Fig. 2
2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography images showed lesion in uterine cervix and no distal metastases. PET = positron emission tomography |
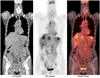 | Fig. 32-[18F] fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) images after radical hysterectomy showed multiple 18F-FDG-avid lesions in mediastinum, liver and left kidney, which were consistent with metastases. |
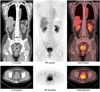 | Fig. 4
2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) images showed huge 18F-FDG-avid mass in posterior pelvis, which was compatible with cervical cancer. Several hypermetabolic lesions in bilateral iliac and retroperitoneal regions were demonstrated, which were mostly related to metastatic lymph nodes. Incidentally, hypermetabolic lesion in left breast was also noted. Biopsy of this lesion showed metastatic neuroendocrine carcinoma. |
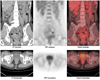 | Fig. 5
2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography images showed lesion in vagina. PET = positron emission tomography |
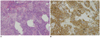 | Fig. 6
This is 28-year-old woman. Histopathological examination (× 100) of tumor showed infiltration of blue round cells with vesicular nuclei, scanty cytoplasma, high nuclear/cytoplasma ratio and extensive necrosis arranged in nest on hematoxylin and eosin stain (A); and positive immunohistochemical stain for synaptophysin (B). These features confirmed diagnosis of neuroendocrine carcinoma. |
Notes
This study was supported by grants from China Medical University Hospital (DMR-100-076 and DMR-100-077), the Taiwan Department of Health Clinical Trial and Research Center for Excellence (DOH101-TD-B-111-004), and the Taiwan Department of Health Cancer Research Center for Excellence (DOH101-TD-C-111-005).
References
1. Oberg K, Castellano D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis Rev. 2011. 30:Suppl 1. 3–7.
2. Albores-Saavedra J, Gersell D, Gilks CB, Henson DE, Lindberg G, Santiago H, et al. Terminology of endocrine tumors of the uterine cervix: results of a workshop sponsored by the College of American Pathologists and the National Cancer Institute. Arch Pathol Lab Med. 1997. 121:34–39.
3. Sato Y, Shimamoto T, Amada S, Asada Y, Hayashi T. Large cell neuroendocrine carcinoma of the uterine cervix: a clinicopathological study of six cases. Int J Gynecol Pathol. 2003. 22:226–230.
4. Krivak TC, McBroom JW, Sundborg MJ, Crothers B, Parker MF. Large cell neuroendocrine cervical carcinoma: a report of two cases and review of the literature. Gynecol Oncol. 2001. 82:187–191.
5. Reig Castillejo A, Membrive Conejo I, Foro Arnalot P, Rodríguez de Dios N, Algara López M. Neuroendocrine small cell carcinoma of the uterine cervix. Clin Transl Oncol. 2010. 12:512–513.
6. McCusker ME, Coté TR, Clegg LX, Tavassoli FJ. Endocrine tumors of the uterine cervix: incidence, demographics, and survival with comparison to squamous cell carcinoma. Gynecol Oncol. 2003. 88:333–339.
7. Wang KL, Yang YC, Wang TY, Chen JR, Chen TC, Chen HS, et al. Neuroendocrine carcinoma of the uterine cervix: a clinicopathologic retrospective study of 31 cases with prognostic implications. J Chemother. 2006. 18:209–216.
8. Chung HH, Nam BH, Kim JW, Kang KW, Park NH, Song YS, et al. Preoperative [18F]FDG PET/CT maximum standardized uptake value predicts recurrence of uterine cervical cancer. Eur J Nucl Med Mol Imaging. 2010. 37:1467–1473.
9. Nakamura K, Okumura Y, Kodama J, Hongo A, Kanazawa S, Hiramatsu Y. The predictive value of measurement of SUVmax and SCC-antigen in patients with pretreatment of primary squamous cell carcinoma of cervix. Gynecol Oncol. 2010. 119:81–86.
10. Yilmaz M, Adli M, Celen Z, Zincirkeser S, Dirier A. FDG PET-CT in cervical cancer: relationship between primary tumor FDG uptake and metastatic potential. Nucl Med Commun. 2010. 31:526–531.
11. Jhingran A, Levenback C. Katz VL, Lentz GM, Lobo RA, Gershenson DM, editors. Malignagnt diseases of the cervix: microinvasive and invasive carcinoma: diagnosis and management. Comprehensive Gynecology. 2007. 5th ed. Philadelpia, PA: Mosby Elsevier.
12. Henriksen E. The lymphatic spread of carcinoma of the cervix and of the body of the uterus; a study of 420 necropsies. Am J Obstet Gynecol. 1949. 58:924–942.
13. Yang DH, Kim JK, Kim KW, Bae SJ, Kim KH, Cho KS. MRI of small cell carcinoma of the uterine cervix with pathologic correlation. AJR Am J Roentgenol. 2004. 182:1255–1258.
14. Okamoto Y, Tanaka YO, Nishida M, Tsunoda H, Yoshikawa H, Itai Y. MR imaging of the uterine cervix: imaging-pathologic correlation. Radiographics. 2003. 23:425–445. quiz 534-535.
15. Yen TC, Ng KK, Ma SY, Chou HH, Tsai CS, Hsueh S, et al. Value of dual-phase 2-fluoro-2-deoxy-d-glucose positron emission tomography in cervical cancer. J Clin Oncol. 2003. 21:3651–3658.
16. Unger JB, Lilien DL, Caldito G, Ivy JJ, Charrier A, Bellaire B. The prognostic value of pretreatment 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography scan in women with cervical cancer. Int J Gynecol Cancer. 2007. 17:1062–1067.
17. Waggoner SE. Cervical cancer. Lancet. 2003. 361:2217–2225.
18. Trinh XB, Bogers JJ, Van Marck EA, Tjalma WA. Treatment policy of neuroendocrine small cell cancer of the cervix. Eur J Gynaecol Oncol. 2004. 25:40–44.
19. Belhocine TZ. 18F-FDG PET imaging in posttherapy monitoring of cervical cancers: from diagnosis to prognosis. J Nucl Med. 2004. 45:1602–1604.
20. Pallardy A, Bodet-Milin C, Oudoux A, Campion L, Bourbouloux E, Sagan C, et al. Clinical and survival impact of FDG PET in patients with suspicion of recurrent cervical carcinoma. Eur J Nucl Med Mol Imaging. 2010. 37:1270–1278.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download