Abstract
Objective
The objective of this study was to evaluate the technical aspects and clinical efficacy of selective embolization for post-endoscopic sphincterotomy bleeding.
Materials and Methods
We reviewed the records of 10 patients (3%; M:F = 6:4; mean age, 63.3 years) that underwent selective embolization for post-endoscopic sphincterotomy bleeding among 344 patients who received arteriography for nonvariceal upper gastrointestinal bleeding from 2000 to 2009. We analyzed the endoscopic procedure, onset of bleeding, underlying clinical condition, angiographic findings, interventional procedure, and outcomes in these patients.
Results
Among the 12 bleeding branches, primary success of hemostasis was achieved in 10 bleeding branches (83%). Secondary success occurred in two additional bleeding branches (100%) after repeated embolization. In 10 patients, post-endoscopic sphincterotomy bleedings were detected during the endoscopic procedure (n = 2, 20%) or later (n = 8, 80%), and the delay was from one to eight days (mean, 2.9 days; ± 2.3). Coagulopathy was observed in three patients. Eight patients had a single bleeding branch, whereas two patients had two branches. On the selective arteriography, bleeding branches originated from the posterior pancreaticoduodenal artery (n = 8, 67%) and anterior pancreaticoduodenal artery (n = 4, 33%), respectively. Superselection was achieved in four branches and the embolization was performed with n-butyl cyanoacrylate. The eight branches were embolized by combined use of coil, n-butyl cyanoacrylate, or Gelfoam. After the last embolization, there was no rebleeding or complication related to embolization.
Bleeding after endoscopic sphincterotomy (ES) is one of the most frequent complications of endoscopic retrograde cholangiopancreatography (ERCP), and has been reported in 1-10% of patients. Most post-ES bleedings stop spontaneously, but significant bleeding may occur during the procedure or several days later. Post-ES bleeding is rarely encountered by interventional radiologists, unlike other nonvariceal upper gastrointestinal bleeding, because various hemostatic procedures are performed simultaneously or repeatedly during the endoscopic procedure. However, in cases of endoscopic hemostasis failure caused by massive bleeding or technical failure, radiologic or surgical hemostasis may be required (1-3).
According to previous reports, since the duodenum has a rich vascular network, rebleeding after embolization is common when the embolization has been performed incompletely or coagulopathy has existed (4, 5). On the other hand, embolization may cause ischemic complications of the duodenum when the gastroduodenal artery is embolized or the patient has had surgically altered anatomy (4, 6). Therefore, selective embolization of bleeding branch for post-ES bleeding is required to reduce rebleeding or ischemic complication related to embolization. However, only a few reports have addressed the endovascular management of post-ES bleeding and these reports have shown limited evaluation for its technical aspect or clinical efficacy due to small study populations or the lack of analysis on post-ES bleeding (3, 4, 7).
Thus, in this study we aimed to retrospectively evaluate the technical aspects and clinical efficacy of selective embolization of post-ES bleeding.
Our Institutional Review Board approved this retrospective study and informed consent from the subjects was waived. We analyzed patient demographics, underlying diseases and clinical conditions of the patients, ES procedure, onset of bleeding, angiographic findings, interventional procedure and outcomes.
Between January 2000 and January 2009, embolizations for nonvariceal upper gastrointestinal bleeding were performed in 344 patients at our institute. Among these, 10 patients (3%; M:F = 6:4; mean age, 63.3 years) underwent embolization because of post-ES bleeding. The causes of sphincterotomy were CBD stone (n = 5), GB cancer (n = 2), CBD stricture (n = 2), and pancreatic cancer (n = 1). ES was performed by standard sphincterotomy (n = 6) or needle knife sphincterotomy (n = 4). Coagulopathy was identified in three patients. No patients had previously received anticoagulation therapy. The characteristics of the patients are shown in Table 1.
Endoscopic retrograde cholangiopancreatography was performed using a TJF-240 or TJF-200 Olympus duodenoscope (Olympus Optical, Tokyo, Japan). All patients were given prophylactic antibiotics and midazolam before the procedure. Standard sphincterotomy was performed with 20 to 30 mm cutting wire length for ES. Pre-cut sphincterotomy was performed with a needle knife. All ES procedures were performed by two experienced pancreaticobiliary endoscopists.
When immediate or delayed ES bleeding occurred, patients underwent an endoscopic hemostatic procedure or angiographic hemostasis according to the severity of the bleeding and the endoscopic accessibility to the bleeding focus.
Two interventional radiologists, with 17 and 28 years of experience, respectively, performed all interventional procedures. Celiac, superior mesenteric artery (SMA), or both site arteriography using a 6.5-Fr or 5-Fr Rösch hepatic catheter or a 5-Fr Cobra-type catheter (Cook, Bloomington, IN) were performed to evaluate the bleeding focus using a digital subtraction angiography unit (Angiostar; Siemens, Erlangen, Germany; V-3000; Philips Medical Systems, Einthoven, The Netherlands, Allura Xper; Philips Medical Systems, Einthoven, The Netherlands). If bleeding was detected on the celiac and/or SMA arteriography, selective arteriography of the anterior or posterior pancreaticoduodenal artery was performed using a 2.3 or 2.0-Fr microcatheter (Microferret, Cook, Bloomington, IN; Progreat, Terumo, Tokyo, Japan) to define the bleeding focus and determine the origin of the responsible branch.
When a single bleeding branch was clearly identified, we attempted to advance the microcatheter carefully into the branch. If the microcatheter was successfully introduced into the bleeding branch, we embolized the branch with n-butyl cyanoacrylate (NBCA). If we failed, bleeding branch embolization was performed by combined use of coil, NBCA, or Gelfoam. For embolization, NBCA was mixed with iodized oil (Lipiodol, Andre Guerbet, Aulnay-Sous-Bois, France) at a ratio of 1:3.
After embolization, completion angiography of the celiac artery or SMA was performed to confirm the absence of bleeding, and when confirmed, the procedure was concluded.
During the hospital period, patients were closely monitored if they exhibited rebleeding or complications related to embolization. After discharge, patients were regularly followed up in the outpatient clinic. During the follow-up period, patients were checked to determine whether there were symptoms or signs indicating late complications related to embolization. The mean follow-up period was 18.1 months (range 0.3 to 48 months).
Of the 12 bleeding branches, primary success of hemostasis was achieved in 10 bleeding branches (83%). Secondary success was achieved in two additional bleeding branches (100%) after repeated embolization.
In 10 patients, post-ES bleedings were detected during the endoscopic procedure (n = 2, 20%) or later (n = 8, 80%), and the delayed time between ES and bleeding in the eight patients ranged from one to eight days (mean, 2.9 days; ± 2.3). Post-ES bleeding was diagnosed by endoscopy (n = 5), computed tomographic (CT) angiography (n = 3), or both modalities (n = 2). Among the eight delayed bleeding patients, one patient experienced immediate bleeding during ES, which stopped after epinephrine spray application and ethanol injection at the bleeding focus during the procedure, but rebleeding occurred one day later. On the endoscopy, oozing type bleeding occurred in four patients whereas arterial pumping occurred in one. In two patients, the bleeding type was not recorded. CT angiography showed contrast material leakage on the arterial phase images in five patients.
Eight patients had a single bleeding branch at the bleeding focus, whereas two patients had two branches (patient 7, 10). Bleeding vessels were detected by conventional angiography of the celiac trunk (n = 5), SMA (n = 5), or both (n = 2). Bleeding branches arose from the posterior pancreaticoduodenal artery (n = 8, 67%) or the anterior pancreaticoduodenal artery (n = 4, 33%). All bleeding branches were vasa recta type vessels that originated from the pancreaticoduodenal artery.
Superselection was achieved in four branches and these were embolized using NBCA (patient 4, 7, 8). The remaining eight branches were embolized using one of three methods: 1) Embolization at just the distal portion of the origin of the bleeding branch with coil and subsequent embolization of the branch with Gelfoam or NBCA (patient 10), 2) Short segmental embolization with a coil or NBCA covering proximal and distal portion of bleeding branch origin (patient 1, 3, 5, 9), and 3) Embolization with Gelfoam at just the proximal portion to the orifice of the bleeding branch with appropriate injection pressure in order to prevent the Gelfoam particles from passing into the opposite collateral arteries (patient 2, 6) (Table 1) (Figs. 1-3).
Of the 10 bleeding branches (83%) with primary success of hemostasis, one bleeding branch of a patient who had two bleeding branches did not show bleeding at first, but instead, showed delayed bleeding five days later caused by aggravation of coagulopathy (patient 7). In two bleeding branches (17%), rebleeding occurred and repeated embolizations were performed (patient 5, 6). One of these was embolized with a coil and NBCA, whereas the other was embolized with Gelfoam particles. In both cases, no further bleeding was detected on completion angiography at the initial session. However, these patients showed hematemesis at the same day. Therefore, the repeated embolizations were performed at the same day in two patients.
During the hospital period, follow up endoscopy was performed in one patient to remove the residual common bile duct (CBD) stone. In three patients, follow up CT was performed to evaluate the disease status. No further bleeding was detected in follow up endoscopy and CT scan.
Mean follow-up period of all patients was 18.1 months (range 0.3 to 48 months). After the last embolization, all patients did not show rebleeding or acute duodenal ischemia or pancreatitis. Two patients expired due to sepsis (patient 3, 7). One of them had received liver transplantation owing to underlying liver cirrhosis and underwent endoscopic plastic stent insertion because of liver transplantation-related CBD stricture (patient 3). The patient expired at 47 days after embolization due to aggravated underlying infection related with immunosuppression. The other patient expired at seven days after embolization due to sepsis accompanied by sphincterotomy site infection and peritonitis (patient 7). One patient expired due to disease progression at 40 days after embolization (patient 10). Two patients underwent pancreaticoduodenectomy due to underlying disease (patient 6, 8). One of these patients underwent pancreaticoduodenectomy with the impression of the malignancy of the CBD, but the final pathology was confirmed as benign CBD stricture (patient 8). There were no symptoms or signs indicating late complication related to embolization such as duodenal stricture in the follow-up period.
Post-ES bleeding is one of the most serious complications of ERCP. Its incidence has been variably reported in other studies, because of the difference in the definition of bleeding (8). Since minor bleeding is common during ERCP and shows a self limiting feature, it is usually not considered as a complication (9). However, clinically significant bleedings have been reported to occur in 2-11% of patients. Among these patients, surgery was necessary in up to 17%, and death occurred in about 0.3% (10-12). Several studies suggest that 4% to 67% of all post-ES bleedings are delayed bleedings, and that one or more subsequent endoscopic procedures were required to control bleeding (10, 11, 13, 14).
Recently, several endoscopic hemostatic methods have been attempted to stop post-ES bleeding. These include the injection of epinephrine or a sclerosing agent, balloon tamponade, fibrin glue injection, hemoclip placement, electrocoagulation, and temporary stent placement (2, 8, 12, 15-18). These endoscopic procedures or combined endoscopic therapies are considered to be the treatments of choice and have high success rates. However, in some cases, massive bleeding occurs during ES and obscures the endoscopic field, which makes subsequent hemostatic procedures technically difficult and may result in the failure of the endoscopic procedure. In these situations, bleeding needs to be stopped by radiologic or surgical methods (3, 10).
According to the report of Yamaguchi et al. (19), the papillary artery originates from the communicating artery which connects the posterior superior pancreaticoduodenal artery (PSPDA) and the anterior superior pancreaticoduodenal artery (ASPDA) (74%), or directly from the posterior pancreaticoduodenal artery as a vasa recta type (26%) among 73 papillary arteries. According to their report, no papillary artery arises from the anterior pancreaticoduodenal artery. Kimura et al. (20) described the vascular anatomy of the pancreaticoduodenal region. They mentioned the papillary artery as a consistent branch of the PSPDA supplying the duodenal papilla. They also suggested that the papillary artery and communicating artery are important during duodenum-preserving pancreatic surgery. According to other reports about the communicating artery, which issues the papillary artery, its incidence is variable (0-100%) and is often confused with a choledochal branch of PSPDA (21, 22). As mentioned above, the origin of the papillary artery was variably reported and such variety of its origin may make it technically difficult to embolize bleeding branches selectively.
Previously reported cases of the angiographic management of post-ES bleeding are rare. Saeed et al. (3) reported three cases of transcatheter embolization for post-ES bleeding. According to their report, gastroduodenal arteries were embolized with Gelfoam and coils to stop the bleeding and hemostasis was successfully achieved without recurrence. Poultsides et al. (4) observed that post-ES bleeding occurred in gastrointestinal hemorrhage in 10 out of 57 patients. However, they did not describe detailed embolization techniques or success rate of hemostasis of post-ES bleeding. Defreyne et al. (7) reported only two cases of post-ES bleeding among 91 patients with nonvariceal gastrointestinal hemorrhage. They embolized bleeding with flow directed delivery of various embolic materials but failed in 50% of patients. In these studies, the technical aspect or clinical efficacy of embolization for post-ES bleeding were not sufficiently evaluated because they analyzed a small population or did not focus on post-ES bleeding.
In our study, 12 bleeding vessels of post-ES bleeding originated from the posterior pancreaticoduodenal artery (n = 8, 67%) or the anterior pancreaticoduodenal artery (n = 4, 33%). According to a previous study, the papillary artery mostly originated from the communicating artery or posterior pancreaticoduodenal artery (19-22). In accordance with a previous study, most bleeding branches arose from the posterior pancreaticoduodenal artery in our cases. Therefore, the posterior pancreaticoduodenal artery should be suspected as a main origin of the bleeding vessel when it is difficult to distinguish the bleeding vessel due to complicated periampullary angiographic findings. In particular, these results must be helpful in making a quick decision on which vessel needs to be catheterized in clinical practice.
Of the 12 bleeding vessels identified in our cohort, bleeding was detected from the celiac (n = 5), SMA (n = 5), or both site arteriography (n = 2). We were able to determine the optimal approach to the bleeding branches by celiac and SMA arteriography. On the angiography, the course of the posterior pancreaticoduodenal artery was shorter than that of anterior pancreaticoduodenal artery and showed early branching appearance from the gastroduodenal artery (23). Although the course from SMA was shorter than from the celiac trunk, it was sometimes easier to approach through the celiac trunk than through the SMA because of an acute angle between the SMA and the inferior pancreaticoduodenal artery. For this reason, we performed celiac and SMA arteriography beforehand in all cases.
During embolization procedures, we were able to embolize the responsible bleeding branch directly without any complications when the branch was successfully selected with a microcatheter. However, when bleeding branches were not selected, problems were experienced because the pancreaticoduodenal artery is a short vascular arcade between the celiac trunk and the SMA, and flow in this vascular segment is highly susceptible to pressure gradient changes. Moreover, in the case of proximal embolization, back-bleeding may occur due to opposite collateral flow (24). Therefore, we used various embolization methods, with combined use of coil, NBCA, and Gelfoam particles under the principle of proximal and distal embolization of the bleeding vessel. We think this principle is especially important during the embolization of vessels with a direct collateral channel like the pancreaticoduodenal artery.
We experienced no duodenal ischemia or duodenal infarction in the patients who did not undergo duodenectomy. According to the experimental study on small bowel ischemia induced by embolization, embolization involving three or fewer vasa recta was relatively tolerable to ischemic bowel damage (25). Therefore, we tried to embolize the bleeding branch as selectively as possible with various techniques. Therefore, we could embolize the responsible branch to less than three and no serious ischemic complication occurred in our cases.
In our cases, two bleeding branches in a patient were found in two cases. Theoretically, multiple bleeding branches at bleeding focus may occur during ES, because there is no anatomic landmark of avascular zone on duodenal papilla. Therefore, possibility of multiple bleeding branches should be considered when the diagnostic arteriography is performed. We experienced recurrent bleeding episode five days after from the branch which did not bleed at initial embolization in one patient. We think this recurrent bleeding occurred because of the progression of the coagulopathy. Rebleeding from the same branch with the previously embolized vessel occurred in two bleeding branches due to incomplete embolization in initial sessions. One of these was embolized with coil and NBCA and the other was embolized with Gelfoam particles. We think that in the former case, despite NBCA not being completely cast at the bleeding branch, bleeding may have ceased in the initial session due to a spasm of the vessel around the bleeding focus. In the latter case, the Gelfoam particles may have migrated or absorbed as time passed. Therefore, we should repeat the sessions at the same day in two patients.
Our study has some limitations that warrant consideration. First, it is limited by its retrospective nature. In particular, medical records were not complete. In two patients, the bleeding pattern was not noted during the initial endoscopy, which could have been problematic when considering the merits of endoscopic or interventional management. Second, our study is limited by its size, and thus, many points should be addressed concerning the technical aspects of embolization as well as vascular supply of the duodenal papilla.
In conclusion, selective embolization is feasible for the management of post-ES bleeding and its clinical efficacy is good to apply in most clinical situations. In particular, the causative vessels of post-ES bleeding mainly arise from the posterior pancreaticoduodenal artery. Therefore, we have to pay attention at first to the posterior pancreaticoduodenal artery in the clinical practice of embolization for post-ES bleeding.
Figures and Tables
Fig. 1
52-year-old male was admitted for benign distal common bile duct stricture and presented with bleeding through endoscopic nasobiliary drainage (patient 8).
A. Celiac arteriography shows extravasation of contrast material (black arrow) from vasa recta of anterior superior pancreaticoduodenal artery (white arrow). Endoscopic nasobiliary drainage was inserted into common bile duct through duodenum (arrowheads). B. Microcatheter was introduced into vasa recta of anterior superior pancreaticoduodenal artery, which was branch responsible for bleeding (white arrows). Tip of microcatheter was wedged into bleeding branch (arrowhead), and n-butyl cyanoacrylate mixture was injected to embolize bleeding branch.
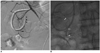
Fig. 2
59-year-old male presented with hematemesis after common bile duct sweeping with stone basket for stone removal (patient 1).
A. Selective arteriography of posterior superior pancreaticoduodenal artery shows active bleeding from single vasa recta (arrow). B. Superior mesenteric arteriography shows no further active bleeding after coil embolization covering bleeding branch orifice (arrow).
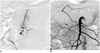
Fig. 3
66-year-old male was admitted for gallbladder cancer and presented with hematemesis and bleeding via endoscopic nasobiliary drainage (patient 10).
A. CT scan shows enhancing mass lesion in confluence level of bile duct that originated from gallbladder (arrow) and upstream bile duct dilatation. B. Celiac arteriography shows extravasation of contrast material from posterior superior pancreaticoduodenal artery (arrow). Endoscopic nasobiliary drainage was inserted in common bile duct through duodenum (arrowheads). C. Selective arteriography of posterior superior pancreaticoduodenal artery shows active bleeding from vasa recta (arrow). D. Completion celiac arteriography shows no evidence of further active bleeding after embolization with Gelfoam particles. Embolization was done at just distal portion of bleeding branch with microcoil.
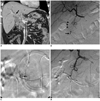
Table 1
Clinical Data of Patients Who Underwent Embolization for Post-ES Bleeding

Note.- CBD = common bile duct, ENBD = endoscopic nasobiliary drainage, ES = endoscopic sphincterotomy, GB = gallbladder, NBCA = n-butyl cyanoacrylate, PDA = pancreaticoduodenal artery
*Two embolization procedures were performed at single branch due to incomplete embolization during initial session.
**Among two bleeding branches, one showed delayed bleeding five days later.
References
1. Ferreira LE, Baron TH. Post-sphincterotomy bleeding: who, what, when, and how. Am J Gastroenterol. 2007. 102:2850–2858.
2. Wilcox CM, Canakis J, Mönkemüller KE, Bondora AW, Geels W. Patterns of bleeding after endoscopic sphincterotomy, the subsequent risk of bleeding, and the role of epinephrine injection. Am J Gastroenterol. 2004. 99:244–248.
3. Saeed M, Kadir S, Kaufman SL, Murray RR, Milligan F, Cotton PB. Bleeding following endoscopic sphincterotomy: angiographic management by transcatheter embolization. Gastrointest Endosc. 1989. 35:300–303.
4. Poultsides GA, Kim CJ, Orlando R 3rd, Peros G, Hallisey MJ, Vignati PV. Angiographic embolization for gastroduodenal hemorrhage: safety, efficacy, and predictors of outcome. Arch Surg. 2008. 143:457–461.
5. Loffroy R, Guiu B, Cercueil JP, Lepage C, Latournerie M, Hillon P, et al. Refractory bleeding from gastroduodenal ulcers: arterial embolization in high-operative-risk patients. J Clin Gastroenterol. 2008. 42:361–367.
6. Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001. 12:195–200.
7. Defreyne L, Vanlangenhove P, De Vos M, Pattyn P, Van Maele G, Decruyenaere J, et al. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001. 218:739–748.
8. Leung JW, Chan FK, Sung JJ, Chung S. Endoscopic sphincterotomy-induced hemorrhage: a study of risk factors and the role of epinephrine injection. Gastrointest Endosc. 1995. 42:550–554.
9. Costamagna G. What to do when the papilla bleeds after endoscopic sphincterotomy. Endoscopy. 1998. 30:40–42.
10. Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996. 335:909–918.
11. Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991. 37:383–393.
12. Sherman S, Hawes RH, Nisi R, Lehman GA. Endoscopic sphincterotomy-induced hemorrhage: treatment with multipolar electrocoagulation. Gastrointest Endosc. 1992. 38:123–126.
13. Kim KO, Kim TN, Kim SB, Lee JY. Characteristics of delayed hemorrhage after endoscopic sphincterotomy. J Gastroenterol Hepatol. 2010. 25:532–538.
14. Gholson CF, Favrot D, Vickers B, Dies D, Wilder W. Delayed hemorrhage following endoscopic retrograde sphincterotomy for choledocholithiasis. Dig Dis Sci. 1996. 41:831–834.
15. Mutignani M, Seerden T, Tringali A, Feisal D, Perri V, Familiari P, et al. Endoscopic hemostasis with fibrin glue for refractory postsphincterotomy and postpapillectomy bleeding. Gastrointest Endosc. 2010. 71:856–860.
16. Shah JN, Marson F, Binmoeller KF. Temporary self-expandable metal stent placement for treatment of post-sphincterotomy bleeding. Gastrointest Endosc. 2010. 72:1274–1278.
17. Kuran S, Parlak E, Oguz D, Cicek B, Disibeyaz S, Sahin B. Endoscopic sphincterotomy-induced hemorrhage: treatment with heat probe. Gastrointest Endosc. 2006. 63:506–511.
18. Matsushita M, Takakuwa H, Shimeno N, Uchida K, Nishio A, Okazaki K. Prophylactic injection of hypertonic saline-epinephrine oral to the papilla for prevention of postsphincterotomy bleeding. J Clin Gastroenterol. 2010. 44:e167–e170.
19. Yamaguchi H, Wakiguchi S, Murakami G, Hata F, Hirata K, Shimada K, et al. Blood supply to the duodenal papilla and the communicating artery between the anterior and posterior pancreaticoduodenal arterial arcades. J Hepatobiliary Pancreat Surg. 2001. 8:238–244.
20. Kimura W, Morikane K, Futakawa N, Shinkai H, Han I, Inoue T, et al. A new method of duodenum-preserving subtotal resection of the head of the pancreas based on the surgical anatomy. Hepatogastroenterology. 1996. 43:463–472.
21. Bertelli E, Di Gregorio F, Bertelli L, Mosca S. The arterial blood supply of the pancreas: a review. I. The superior pancreaticoduodenal and the anterior superior pancreaticoduodenal arteries. An anatomical and radiological study. Surg Radiol Anat. 1995. 17:97–106.
22. Bertelli E, Di Gregorio F, Bertelli L, Civeli L, Mosca S. The arterial blood supply of the pancreas: a review. II. The posterior superior pancreaticoduodenal artery. An anatomical and radiological study. Surg Radiol Anat. 1996. 18:1–9.
23. Hong KC, Freeny PC. Pancreaticoduodenal arcades and dorsal pancreatic artery: comparison of CT angiography with three-dimensional volume rendering, maximum intensity projection, and shaded-surface display. AJR Am J Roentgenol. 1999. 172:925–931.
24. Jae HJ, Chung JW, Jung AY, Lee W, Park JH. Transcatheter arterial embolization of nonvariceal upper gastrointestinal bleeding with N-butyl cyanoacrylate. Korean J Radiol. 2007. 8:48–56.
25. Jae HJ, Chung JW, Kim HC, So YH, Lim HG, Lee W, et al. Experimental study on acute ischemic small bowel changes induced by superselective embolization of superior mesenteric artery branches with N-butyl cyanoacrylate. J Vasc Interv Radiol. 2008. 19:755–763.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download