Abstract
Castleman's disease is an uncommon disorder characterized by benign proliferation of the lymphoid tissue that occurs most commonly in the mediastinum. Although unusual locations and manifestations have been reported, involvement of the renal parenchyma and sinus, and moreover, manifestations as cardiac tamponade are extremely rare. Here, we present a rare case of Castleman's disease in the renal parenchyma and sinus that also accompanied cardiac tamponade.
Castleman's disease is a rare lymphoproliferative disease that is also known as angiofollicular lymphoid nodular hyperplasia or giant lymphoid nodular hyperplasia. This condition most commonly occurs within the thorax, although rare extrathoracic presentations have been described (1). Castleman's disease in the renal parenchyma or sinus is extremely rare. In addition, cardiac tamponade secondary to Castleman's disease is even rarer.
To the best of our knowledge, there have been no reports of Castleman's disease simultaneously presenting as renal parenchymal, sinus tumors, and cardiac tamponade. Here we describe our experience with one such patient.
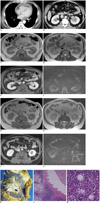
A 59-year-old man who had exertional dyspnea for 2 weeks was admitted to the hospital. His history was unremarkable. Laboratory findings revealed mild anemia (hemoglobin, 10.3 g/dL; hematocrit, 31.9%) and a high C-reactive protein level (9.85 mg/dL). During the work-up, chest and abdominal computed tomography (CT) revealed mild enhancement of a soft-tissue mass in the left renal sinus as well as moderate pericardial effusion (Fig. 1A, B). Transthoracic echocardiography showed a moderate amount of pericardial effusion compressing the entire heart as well as a thickened pericardium. Pericardiocentesis was performed and 500 mL of hemorrhagic fluid was aspirated. Cytology of the fluid was showed no malignant cells, and histological examination of the pericardium revealed nonspecific fibrinous pericarditis.
Magnetic resonance imaging (MRI) of the left renal sinus mass (Fig. 1C, D) revealed homogeneous tissue and showed isointense signals on T1-weighted images as well as hypointense signals on T2-weighted images relative to those in the renal cortex. Renal parenchymal masses (Fig. 1G, H) showed isointense signals on T1-weighted images and T2-weighted images. Mild contrast enhancement of the renal sinus tumor was noted (Fig. 1E). We could not detect the renal parenchymal masses on contrast-enhanced CT and MRI (Fig. 1I) because they were similarly enhanced as the renal parenchyma. Diffusion-weighted imaging (b = 1000 s/mm2) showed that the renal sinus and parenchymal masses had high signal intensity (Fig. 1F, J). However, urinalysis findings were within normal limits and urine cytology showed no evidence of malignancy. Nevertheless, we suspected the masses were malignant types such as transitional cell carcinoma or malignant lymphoma.
The patient underwent left nephrectomy that revealed white solid masses in the left renal parenchyma and sinus (Fig. 1K). Histological examination (Fig. 1L, M) revealed hyperplastic lymphoid follicles in the renal parenchyma and sinus; in addition, the interfollicular areas contained sheets of plasma cells. These histological findings were consistent with the plasma cell type of Castleman's disease. Twenty days after tumor resection, laboratory findings yielded hypergammaglobulinemia (29.1%) and an elevated erythrocyte sedimentation rate (ESR; 105 mm/h).
Castleman's disease remains a rare and poorly understood disease characterized by massive growth of the lymphoid tissue. This condition was first described by Castleman and Towne (2) as a localized mediastinal lymph node hyperplasia resembling thymoma. The pathogenesis of Castleman's disease remains unknown. Chronic low-grade inflammation, an immunodeficiency state, and autoimmunity have been proposed as probable mechanisms.
Pathologically, Castleman's disease can be classified as a hyaline vascular, plasma cell, or mixed type (3) and clinically as a unicentric or multicentric type (4). The most common histological type is hyaline vascular, which is characterized by small hyaline vascular follicles and interfollicular capillary proliferation. This type is present in 90% of patients with Castleman's disease and appears predominantly in the thorax. It primarily affects adults < 30 years of age and is usually asymptomatic.
The plasma cell type, characterized by large follicles with intervening sheets of plasma cells with less vascularity than the hyaline vascular type, is associated with many signs and symptoms. These patients present with fever, malaise, sweating, weight loss, anemia, thrombocytosis, hypergammaglobulinemia, and an elevated ESR evidenced by B-cell hyperreactivity. Our patient had anemia, hypergammaglobulinemia, and an elevated ESR. The mixed histological type is characterized by a combination of hyaline vascular and plasma cell morphology.
Of the two clinical types, the unicentric form is usually found in young people, showing the hyaline vascular pattern in 70-90% of cases, arising in asymptomatic patients or associated with systemic manifestations (especially in plasma cell cases). The less common multicentric form involves more than one disease site. It is typically reported to occur in older individuals and shows a plasma cell pattern in 80-90% of cases. This variant is clinically aggressive with systemic symptoms, hepatosplenomegaly, and generalized lymphadenopathy. Surgery is the treatment of choice for unicentric Castleman's disease, whereas chemotherapy, radiotherapy, and steroids are proposed for inoperable cases and the multicentric type due to its aggressive course (5).
Although Castleman's disease usually involves the mediastinal lymph nodes, it may occur in other areas of the body where lymph nodes are normally found, such as the abdomen, including the intraperitoneal and retroperitoneal regions. The renal parenchyma and sinus are extremely unusual locations for Castleman's disease. The exact mechanism of the development of Castleman's disease in the kidney, which does not normally contain lymphoid tissue, has been not reported in the literature. We think that the mechanism is similar to the assumed mechanism of lymphoma development in the kidney: the tumor may arise from the lymphatic-rich renal capsule or the perinephric fat and invade the parenchyma, or it can arise from lymphocytes present in areas of chronic inflammation (6-8).
There are only a few published articles about Castleman's disease occupying the renal sinus (9, 10) and renal parenchyma (11-14). In addition, a variety of imaging findings of Castleman's disease have been described that can vary by histological type. According to published reports, mild enhancement and hypointensity on T2-weighted images have been described in the plasma cell type (10, 15), whereas marked enhancement and hyperintensity have been seen on T2-weighted images in the hyaline vascular type (16). These findings reflect the hypovascular nature of the plasma type of Castleman's disease.
Nishie et al. (10) reported several instances of Castleman's disease in the renal sinus (five lesions in three patients; the mixed form in one patient and the plasma cell type in two patients), which were unilateral or bilateral, soft, and homogeneous; showed little mass effect and enhancement; and were hypointense compared to the renal cortex on T2-weighted images as seen in our case. Also, in the case of the plasma cell type of Castleman's disease involving the renal parenchyma (14), the renal parenchymal masses were homogeneous and had hypointense signals compared to the renal cortex on T2-weighted images. We believe that mild enhancement and hypointensity on T2-weighted images can be helpful in diagnosing the plasma cell type of Castleman's disease.
To the best of our knowledge, characteristic diffusion-weighted findings of Castleman's disease have not been reported. Oida et al. (17) reported hyperintensity on diffusion-weighted images of retroperitoneal Castleman's disease, a finding that is similar to our case. We think that this hyperintensity can be attributed to the high cellularity of the lesions. In addition, better contrast between the renal parenchymal tumors and the surrounding tissue on diffusion-weighted images was demonstrated in our case, making detection of the tumors more quick and easy. Future studies of diffusion-weighted imaging findings of Castleman's disease are required to further assess this finding.
The differential diagnosis of synchronous renal parenchymal and sinus tumors includes transitional cell carcinoma (TCC), lymphoma, and IgG4-related disease. Radiological findings of renal TCC include a sessile or flat solid mass in the renal calyx and/or pelvis, focal renal pelvic wall thickening, or an infiltrating mass. In cases of renal parenchymal involvement, an infiltrating pattern that obliterates the renal sinus fat and extends into the surrounding parenchyma is seen. TCC is usually associated with a greater degree of obstruction of the collecting system in contrast to Castleman's disease (18). Of the three diagnoses, lymphoma and IgG4-related disease are more difficult than TCC to distinguish from Castleman's disease. Lymphoma can involve the renal parenchyma and sinus. It also has lower signal intensity than does a normal cortex with T1-weighted sequences, and it is relatively iso- or hypointense in T2-weighted sequences (18). In addition, lymphoma is are less enhanced than the renal parenchyma by intravenous administration of iodinated and gadolinium-based contrast material. As a result of the tumor's pliable nature, the resulting hydronephrosis is often mild. The presence of retroperitoneal adenopathy can be useful for distinguishing between lymphoma and Castleman's disease (18). Takahashi et al. (19) described CT and MRI findings of the renal involvement of IgG4-related disease. In that study, renal parenchymal lesions showed decreased enhancement and appeared as small peripheral cortical nodules, round or wedge-shaped lesions, or diffuse patches. Also, the lesions were hypointense on T2-weighted images. Soft-tissue masses in the renal sinuses were described in a previous case report (20). High serum IgG4 concentrations, favorable responses to steroid therapy, and synchronous or metachronous associations with IgG4-related disease in other organs, particularly the pancreas, can be helpful findings that are suggestive of IgG4-related disease (21).
Unusual clinical features associated with Castleman's disease include peripheral neuropathy, myasthenia gravis, growth retardation, erosive stomatitis and keratitis, nephrotic syndrome, amyloidosis, thrombotic thrombocytopenia purpura, myelofibrosis, cardiac tamponade, and recurrent pleural effusion. Cardiac tamponade secondary to Castleman's disease is extremely rare, with only two cases having been reported in the literature (22, 23). According to Nicolosi et al. (22), pericardial effusion can result from impaired reabsorption by the inflamed serous membrane occurring as one manifestation of the generalized inflammatory syndrome related to plasma cell histology. Our case showed fibrinous pericarditis with pericardial effusion as suggested by Nicolosi et al. (22).
Preoperative diagnosis is often difficult since Castleman's disease can occur anywhere lymphoid tissue is found and demonstrates different clinical and radiological findings according to histological type. This case is interesting because of its unusual location and manifestations. To the best of our knowledge, this is the first case of Castleman's disease involving the renal parenchyma and sinus with cardiac tamponade. Although rare, we suggest that the plasma cell type of Castleman's diseases should be added to the differential diagnosis, especially when renal parenchymal and sinus tumors show homogeneity, little mass effect, and enhancement and hypointensity on T2-weighted images. Awareness that cardiac tamponade is one of the manifestations of Castleman's disease may also be helpful in diagnosis.
Figures and Tables
Fig. 1
Plasma cell type of Castleman's disease involving renal parenchyma and sinus in 59-year-old man.
A. Axial contrast-enhanced computed tomography (CT) scan obtained at level of ventricles shows pericardial effusiod producing abnormal flattening of anterior surface of heart (arrow) suggestive of cardiac tamponade. B. Axial contrast-enhanced CT scan obtained at level of renal hilum shows mildly homogenous enhancement of soft-tissue mass (arrows) in left renal sinus. Due tothes mass, renal sinus had mild effect on renal pelvis, causing mild hydronephrosis. C. T1-weighted magnetic resonance (MR) image (TR/TE, 117/4.9) shows soft-tissue mass in left renal sinus (open arrow) with isointense signal compared to that of renal cortex. D. T2-weighted MR image (800/80) shows soft-tissue mass in left renal sinus (open arrow) with slightly hypointense signals compared to that of renal cortex. E. Gadolinium-enhanced T1-weighted MR image shows mild contrast enhancement. F. Diffusion-weighted MR image (b = 1000 s/mm2) shows that mass (open arrow) is mildly hyperintense. G. T1-weighted MR image (TR/TE, 117/4.9) shows contour-bulging masses of renal parenchyma (open arrows) with isointense signals compared to that of renal cortex. H. T2-weighted MR image (800/80) shows contour-bulging masses of renal parenchyma (open arrows) with isointense signals relative to those of renal cortex. I. Gadolinium-enhanced T1-weighted MR image shows contrast enhancement with similar degree as renal parenchyma (open arrows). J. Diffusion-weighted MR image (b = 1000 sec/mm2) shows that masses (open arrows) are mildly hyperintense. K. Photograph of sectioned gross specimen shows whitish tumors (arrows) in renal sinus fat tissue and renal parenchyma. L. Photomicrograph (original magnification, × 40; hematoxylin-eosin stain) shows hyperplastic lymphoid follicles (asterisks) in renal sinus fat tissue below normal renal parenchyma and pelvis in left upper corner. M. Interfollicular areas show heavy infiltration with mature plasma cells (original magnification, × 400; hematoxylin-eosin stain).
References
1. Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972. 29:670–683.
2. Castleman B, Towne VW. Case records of the Massachusetts General Hospital: Case No. 40231. N Engl J Med. 1954. 250:1001–1005.
3. Flendrig JA. Clark RL, Cumley RW, editors. Benign giant lymphoma: clinicopathologic correlation study. The year book of cancer. 1970. Chicago: Year Book Medical Publishers;296–299.
4. McCarty MJ, Vukelja SJ, Banks PM, Weiss RB. Angiofollicular lymph node hyperplasia (Castleman's disease). Cancer Treat Rev. 1995. 21:291–310.
5. Weisenburger DD, Nathwani BN, Winberg CD, Rappaport H. Multicentric angiofollicular lymph node hyperplasia: a clinicopathologic study of 16 cases. Hum Pathol. 1985. 16:162–172.
6. Stallone G, Infante B, Manno C, Campobasso N, Pannarale G, Schena FP. Primary renal lymphoma does exist: case report and review of the literature. J Nephrol. 2000. 13:367–372.
7. Karadeniz C, Oguz A, Ataoglu O, Citak C, Buyan N, Pinarli G, et al. Primary renal lymphoma and xanthogranulomatous pyelonephritis in childhood. J Nephrol. 2002. 15:597–600.
8. Cupisti A, Riccioni R, Carulli G, Paoletti S, Tognetti A, Meola M, et al. Bilateral primary renal lymphoma treated by surgery and chemotherapy. Nephrol Dial Transplant. 2004. 19:1629–1633.
9. Nagahama K, Higashi K, Sanada S, Nezumi M, Itou H. [Multicentric Castleman's disease found by a renal sinus lesion: a case report]. Hinyokika Kiyo. 2000. 46:95–99.
10. Nishie A, Yoshimitsu K, Irie H, Aibe H, Tajima T, Shinozaki K, et al. Radiologic features of Castleman’s disease occupying the renal sinus. AJR Am J Roentgenol. 2003. 181:1037–1040.
11. De Feudis L, Carota G, Sargiacomo R, Traisci G. [Castleman's disease with isolated renal location: clinical case]. Ann Ital Med Int. 1998. 13:117–120.
12. Mah NA, Peretsman SJ, Teigland CM, Banks PM. Castleman disease of the hyaline-vascular type confined to the kidney. Am J Clin Pathol. 2007. 127:465–468.
13. Hatano K, Fujita S, Tsujimoto Y, Takada T, Honda M, Tsujimoto M, et al. Rare case of the hyaline vascular type of Castleman's disease of the kidney. Int J Urol. 2007. 14:1098–1100.
14. Zhu YC, Huang Y, Yao J, Li X, Zhao S, Wei Q, et al. A rare case of Castleman's disease of plasma cell type within kidney. Chin Med J (Engl). 2009. 122:2396–2398.
15. Soler R, Rodríguez E, Bello MJ, Alvarez M. Pancreatic Castleman's disease: MR findings. Eur Radiol. 2003. 13:Suppl 4. L48–L50.
16. Mangini M, Aiani L, Bertolotti E, Imperatori A, Rotolo N, Paddeu A, et al. Parapancreatic Castleman disease: contrast-enhanced sonography and CT features. J Clin Ultrasound. 2007. 35:207–211.
17. Oida Y, Shimizu K, Mukai M, Imaizumi T, Nakamura M, Makuuchi H. FDG-PET and diffusion-weighted MR imaging appearance in retroperitoneal Castleman's disease: a case report. Clin Imaging. 2008. 32:144–146.
18. Sheth S, Ali S, Fishman E. Imaging of renal lymphoma: patterns of disease with pathologic correlation. Radiographics. 2006. 26:1151–1168.
19. Takahashi N, Kawashima A, Fletcher JG, Chari ST. Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology. 2007. 242:791–801.
20. Fukukura Y, Fujiyoshi F, Nakamura F, Hamada H, Nakajo M. Autoimmune pancreatitis associated with idiopathic retroperitoneal fibrosis. AJR Am J Roentgenol. 2003. 181:993–995.
21. Pannala R, Chari ST. Autoimmune pancreatitis. Curr Opin Gastroenterol. 2008. 24:591–596.
22. Nicolosi AC, Almassi GH, Komorowski R. Cardiac tamponade secondary to giant lymph node hyperplasia (Castleman's disease). Chest. 1994. 105:637–639.
23. Zhang C, Miao Q, Chen G, Liu X, Ma G, Cao L, et al. Pericardial tamponade secondary to Castleman's disease. Ann Thorac Surg. 2009. 88:2039.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download