Abstract
Local treatment for hepatocellular carcinoma (HCC) has been widely used in clinical practice due to its minimal invasiveness and high rate of cure. Percutaneous radiofrequency ablation (RFA) is widely used because its treatment effectiveness. However, some serious complications can arise from percutaneous RFA. We present here a rare case of hemorrhagic cardiac tamponade secondary to an anterior cardiac vein (right marginal vein) injury during RFA for treatment of HCC.
Local treatment for hepatocellular carcinoma (HCC) has been widely used in clinical practice due to its minimal invasiveness and high rate of cure; percutaneous options for local treatment include radiofrequency ablation (RFA), ethanol injection, microwave coagulation and laser ablation therapy. Among these, percutaneous RFA is more widely applied because it requires fewer treatment sessions and yields a larger coagulation volume (1). Some serious RFA complications such as a liver abscess, intraperitoneal hemorrhage, biloma, ground pad burn, diaphragmatic injury, pneumothorax, pleural effusion, bowel perforation, hepatic infarction, renal infraction and tumor seeding have been reported (2). The prevalence of major complications from a large multicenter study was found to be 1.5-2.4%, and the mortality rate between 0.09-0.11% (2). We present here a rare case of hemorrhagic cardiac tamponade secondary to an anterior cardiac vein (right marginal vein) injury during RFA for treatment of HCC.
A 56-year-old Malay man with chronic hepatitis B, not on regular follow-up, who first presented to a private medical center with abdominal distension in July 2010, was diagnosed with hepatocellular carcinoma and referred to our institute for further management.
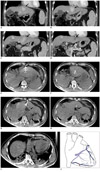
At our institute, a dynamic CT scan of the liver revealed five hypervascular lesions at segments IVa, VII and VIII in the background of cirrhosis. The largest lesion at segment IVa, measured 4.3 × 3.5 cm and associated splenomegaly and esophageal varices but no ascites was observed (Fig. 1A-D).
Local treatment of the multicentric HCC using RFA was planned in agreement with the patient. During the pre-procedural assessment, the patient was incidentally found to have a small ventricular septal defect with left to right shunting. However, the chamber sizes and left ventricular ejection fraction were normal.
At the time of treatment, he had Child Class A compensated liver disease with the following laboratory test results: serum albumin, 32 g/L; serum bilirubin, 21 µmol/L; alanine aminotransferase (ALT), 45 IU/L; aspartate aminotransferase (AST), 54 IU/L; platelet count, 61 × 109/L; International Normalised Ratio, 1.2; and alpha fetoprotein (AFP), 23.8 ng/mL.
The procedure was performed under general anesthesia and fluoroscopic CT guidance (Somatom Definition AS, Siemens Medical Solutions, Erlangen, Germany) using a 10 cm long expandable 15 G StarBurst XL radiofrequency (RF) needle (RITA Medical System, Mountain View, CA, USA) with an array diameter of 5 cm. The RF current generator used was the 1500X RF generator system (RITA Medical System, Mountain View, CA, USA). Four grounding pads were placed on the patient's thighs. The patient's pulse-oximetry, arterial blood pressure, and cardiac activity were monitored during the entire procedure. Under CT fluoroscopic guidance, the largest tumour in segment IVa was identified. The needle tip was placed such that the thines at 2 cm deployment covered the outer margins of the tumour. Deployment was monitored under CT fluoroscopic guidance. This lesion was ablated to 5 cm after which the needle was repositioned twice to ablate the medial and lateral margins to provide a 5 mm margin around the tumour. The entire process was constantly monitored using CT fluoroscopy. The area of coagulation necrosis was seen within the tumour (Fig. 1E-H) with no evidence of any pericardial fluid. During the attempt to remove the RF needle from segment IVa, the thines would not retract. Significant force was finally used to retract the thines.
The operator then decided to ablate the lesion in segment VI. During placement of the needle, it was noted that there was an expanding pericardial effusion (Fig. 1I). The procedure was aborted to facilitate the management of the cardiac tamponade. Immediate pericardiocentesis performed by a cardiologist, was noted to be haemorrhagic. Approximately 300 mL of blood was aspirated but the patient was persistently hypotensive and an emergency sternostomy was performed. Intra-operatively, two litres of blood was evacuated from the pericardium. A puncture wound was noted at the anterior cardiac vein (right marginal vein) (Fig. 1J), which could have led to the haemorrhagic cardiac tamponade. In addition, inflammatory changes are also seen in the adjacent diaphragm but no evidence of any ablation change was seen in the pericardium. The anterior cardiac vein (right marginal vein) was repaired and haemostasis was secured.
The patient was stable post-surgery. While in the intensive care unit, his condition gradually deteriorated with the development of liver failure, upper gastrointestinal bleeding, pneumonia, and sepsis. The patient expired 19 days after the primary event.
Percutaneous RFA is commonly used as local treatment for HCC nodules adjacent to the diaphragm. Haemorrhagic cardiac tamponade is a rare complication. To our knowledge, there are only two cases reported, one by Moumouh et al. (3) and another one by Gao et al. (4).
In our patient, the haemorrhagic cardiac tamponade was secondary to the direct puncture of the RFA needle through the diaphragm and injuring the anterior cardiac vein during an attempt to remove the RFA needle from segment IVa of the liver. We had to be aware that the expandable RFA needle was sometimes difficult to remove after repeated ablations.
To reduce the rate of complication, three important strategies including prevention, early detection, and proper management were acknowledged (2); prevention, was the most important of the three. In this study, four points had to be emphasizedas follows: 1) pre-procedural assessment to identify high risk patients and to optimize them before the procedure, 2) deployment of thermal protection technique for lesions in closed proximity to vulnerable organs 3) use of real-time imaging guidance (e.g., ultrasound or CT fluoroscopy) for placement of the needle prior to ablation and monitoring the position of the deployed thines during ablation as well as during removal of the RFA needle 4) prevention of charring and tissue adhesion of the RFA needle by removal of the RFA needle between treatment cycles to clean the thines and thus reduce the risk of thines getting stuck.
Pre-procedural assessment is important to identify the high risk group such as patients with coagulopathy and poor hepatic reserve. For patients with coagulopathy, the RFA should be postponed until the coagulation profile is corrected. For patients with a poor hepatic reserve, any potential hepatotoxic drug should be withheld and the general condition of the patient optimized prior to the procedure.
Many protective techniques have been developed to thermally insulate and protect the organs at risk. They include using fluid, gas, or balloon interpositions between the organs at risk and the ablation zone. Chen et al. (5) reported that injection of a 5% dextrose solution into the peritoneal cavity reduces the risk of thermal injury to the diaphragm or the bowel during RFA. Bowel protection with balloon interposition during RFA of HCC was reported by Yamakado et al. (6). Buy et al. (7) also demonstrated that CO2 dissection is an effective technique to protect the organs at risk during RFA or cryoablation. All these techniques are increasing the space between the organs at risk and the RFA target area. Therefore, besides providing thermal protection, they can also reduce the risk of direct puncture of adjacent organs by the RFA needle during insertion and removal of the needle.
Real-time imaging such as ultrasound or CT fluoroscopy is useful for monitoring the needle position throughout the RFA. It is important for accurate placement of the needle prior to ablation. It is also crucial for monitoring the position of the deployed thines during ablation as well as during the removal of the RFA needle. Choice of real-time imaging monitoring depends on the local availability. Ultrasound is the most affordable real-time imaging, but it has the disadvantage of poor visualization if there is a gaseous structure or bone around the target lesion. CT fluoroscopy overcomes the limitations of ultrasound, but with the disadvantage of radiation. Proper use of real-time imaging in monitoring the RFA needle position will prevent injury of adjacent organs due to the malposition of the RFA needle.
Prevention of charring and tissue adhesion to the RF needle is not only important to ensure an effective ablation of the lesion, but also to avoid injury to the adjacent organs during withdrawal of the RF needle. A multi-pronged RF needle like the RITA Medical System Starburst XL is known to be relatively difficult to remove (8), especially after prolonged or repeated ablation. This is caused by the charring and tissue adhesion of the RF needle. It may also cause unintentional injury to the adjacent organ during removal. We believe that the difficulty in retracting the thines was the reason for the cardiac tamponade in the case presented. Therefore, it is recommended to frequently remove, clean and redeploy the RF needle to prevent charring and tissue adhesion if a prolonged or repeated ablation of a single lesion is contemplated.
The second strategy in minimizing major complications is early detection. Although early detection cannot reduce the frequency of complications, it can potentially minimize their clinical magnitude. Therefore, the operator and medical personnel who are involved in the care of the patient during and after RFA should be knowledgeable on the spectrum of various RFA complications, so that these complications can be detected early. Close monitoring of the patient during the procedure will help the operator detect the complications early and stop the ablation to prevent more serious damage. Close monitoring of vital signs with a complete blood cell count and measurement of prothrombin time after the procedure is essential for early detection of complications. Immediate (within 24 hours) follow-up CT is a reliable modality for detecting any complications after RFA (2).
The last key strategy is proper management of the complication. Proper management at the appropriate time is obviously an important issue because a complication is not a static condition. Even if the complication is detected early, inappropriate management can result in mortality. Thus, the operator should treat patients with complications properly on the basis of the unique clinical characteristics of each complication.
In summary, we present a case of haemorrhagic cardiac tamponade, which is a rare complication of percutaneous RFA for HCC. As with this patient, the end result was one of fatality. Thus, the operator should exercise all necessary care when embarking on the procedure being fully aware of all the potential complications and taking preventive measures to avoid them.
Figures and Tables
Fig. 1
Hemorrhagic cardiac tamponade secondary to RFA in 56-year-old HCC patient.
A-D. Dynamic CT scan of liver showed ill-defined hypervascular HCC nodules in segment Iva, which is in close proximity to diaphragm and right ventricle (black arrow). E, F. Position of expandable RFA needle in largest HCC nodule in segment IVa. G, H. After 1 cycle of RFA, coagulation necrosis is seen within lesion (black arrow). HCC = hepatocellular carcinoma, RFA = radiofrequency ablation, INR = International Normalised Ratio, RF = radiofrequency. I. Plain CT of post-RFA at lower thorax. Haemorrhagic cardiac tamponade (white asterisk) is noted in post-RFA CT when patient developed hypotension. J. Line art to illustrate location of puncture wound (red dot) of anterior cardiac vein (black arrow). RFA = radiofrequency ablation
References
1. Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004. 127:S159–S166.
2. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003. 23:123–134. discussion 134-136.
3. Moumouh A, Hannequin J, Chagneau C, Rayeh F, Jeanny A, Weber-Holtzscherer A, et al. A tamponade leading to death after radiofrequency ablation of hepatocellular carcinoma. Eur Radiol. 2005. 15:234–237.
4. Gao J, Sun WB, Tong ZC, Ding XM, Ke S. Successful treatment of acute hemorrhagic cardiac temponade in a patient with hepatocellular carcinoma during percutaneous radiofrequency ablation. Chin Med J (Engl). 2010. 123:1470–1472.
5. Chen EA, Neeman Z, Lee FT, Kam A, Wood B. Thermal protection with 5% dextrose solution blanket during radiofrequency ablation. Cardiovasc Intervent Radiol. 2006. 29:1093–1096.
6. Yamakado K, Nakatsuka A, Akeboshi M, Takeda K. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol. 2003. 14:1183–1186.
7. Buy X, Tok CH, Szwarc D, Bierry G, Gangi A. Thermal protection during percutaneous thermal ablation procedures: interest of carbon dioxide dissection and temperature monitoring. Cardiovasc Intervent Radiol. 2009. 32:529–534.
8. Steinke K, King J, Glenn D, Morris DL. Percutaneous radiofrequency ablation of lung tumors: difficulty withdrawing the hooks resulting in a split needle. Cardiovasc Intervent Radiol. 2003. 26:583–585.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download