Abstract
Objective
To report our initial experience on the technical feasibility and safety for hemostasis of a new pneumatic compression device in patients undergoing femoral arteriotomy.
Materials and Methods
This study included 40 consecutive patients in whom hemostasis after transfemoral catheterization was readered by using a pneumatic compression device consisting of an inflatable bulb-containing main body and four pieces of supplementary tape. Medical records were retrospectively reviewed for outcomes and complications of hemostasis. Technical success was defined as achieving immediate hemostasis 10 minutes after applying the device over the arteriotomy sites, and clinical success was defined as the ability to ambulate after 4 hours of bed rest without any complications.
Results
Technical and clinical success was achieved in 38 (95%) and 37 (93%) patients, respectively. In two patients, hemostasis was achieved after conversion to manual compression. One patient required sand bag placement after removal of the device to control minimal oozing of blood. No patients had late complications.
As the need for less invasive therapy increases, percutaneous endovascular procedures are increasingly performed as an alternative to surgery. The common femoral artery is the mainstay for arterial access used for endovascular procedures, as the common femoral artery allows reliable and easy access in most cases, with reasonably consistent hemostasis after the procedure. Although manual compression has classically been the principal method for achieving hemostasis for femoral arteriotomy sites, manual compression increases personnel demands and necessitates prolonged bed rest and patient discomfort. To overcome the disadvantages of manual compression, arterial closure devices (ACDs) have been developed and are increasingly used for achieving hemostasis in arteriotomy sites. Currently, most of the commonly used ACDs are either collagen plug-type or suture-mediated devices (1). However, the safety of ACDs remains in question and they may increase the risks of infection and leg ischemia because most ACDs place foreign bodies inside the artery and tissue track (2-7). Moreover, there are concerns that ACDs may increase the risk of hematomas and pseudoaneurysm formation compared with standard manual compression (8, 9).
A pneumatic compression device is an external compression device which usually involves a small inflatable balloon or bulb, and compression to the femoral arteriotomy occurs via the pressure exerted by the inflated balloon (10-13). Presumably, the use of a pneumatic compression device is not associated with an increased risk of infection or leg ischemia given that nothing is left inside the artery or tissue track. However, there is a relative paucity of research on this type of device compared with the extensive body of information on collagen plug-type or suture-mediated devices.
The purpose of this study was to report our initial experience on the technical feasibility and safety of a new pneumatic compression device (GH-150; Kyung-Won Medical, Seoul, Korea).
Our Institutional Review Board did not require approval of patients or patient informed consent for this retrospective study and included 40 consecutive patients in whom hemostasis of femoral arteriotomy sites was achieved using the GH-150 device over a 6-month period between October 2009 and April 2010 at our cancer center. All patients underwent transarterial chemoembolization for the treatment of hepatocellular carcinoma (n = 39) or transarterial embolization for the control of bleeding which occurred after radiofrequency ablation of an intrahepatic metastatic lesion resulting from rectal cancer (n = 1). An international normalized ratio (INR) < 1.5 and a platelet count > 50,000/µl were required to perform a percutaneous arteriotomy according to our protocol. The patient characteristics and interventional procedural details are summarized in Table 1. The exclusion criteria for application of the device included severe peripheral vascular disease, marked obesity, an arterial sheath > 7 Fr, and patients who declined to provide informed consent for the use of the device. Fifteen patients had a prolonged prothrombin time (INR > 1.2) and/or four patients received transfusions of platelet concentrates to maintain platelet counts > 50,000/µl.
The GH-150 is a single-use, disposable device and consists of a cruciform main body and four pieces of supplementary tape. The main body has a self-adhesive peel backing and a semi-compliant inflatable bulb (4 cm in diameter) under a transparent plastic dome at the center of the body and supplementary tapes also have a self-adhesive peel backing.
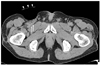
In brief, hemostasis was achieved as follows: With the sheath in the femoral artery, the main body was secured over the puncture site after removal of the self-adhesive backing while positioning the center of the bulb 1 cm proximal to the sheath entry site. Supplementary tapes were applied over the wings of the main body to reinforce the attachment of the main body to the skin (Fig. 1). Then, the bulb was inflated with room air (approximately 150 ml in volume) and the sheath was removed while keeping manual compression over the bulb. Then, manual compression was gradually released over a few seconds under visual inspection for any bleeding below the plastic dome. If any signs of bleeding were present, the bulb was inflated with an additional 20-30 cc of room air. The device was applied for a set time of 10 minutes, which was the routine set time for manual compression at our institution. When any signs or symptoms of leg ischemia developed during the set time of the device application, the bulb was slowly deflated until the ischemic signs or symptoms disappeared, while maintaining control of bleeding. After 10 minutes, the device was deflated and hemostasis was confirmed by viewing the puncture site through the deflated bulb. If hemostasis was successful, the bulb was lightly re-inflated with 70-80 ml of room air and the patient was returned to the ward with the device kept in place. After 4 hours of bed rest, the device was finally removed and the groin was checked for any bleeding or hematoma formation before ambulation. Pulsation of the ipsilateral dorsalis pedis was checked every 30 minutes for two hours and then hourly until the device was removed.
We reviewed medical records for the outcomes and complications of hemostasis. Technical success was defined as achieving immediate hemostasis after the 10-minute application of the device without the need for additional manual compression or other means of achieving hemostasis. Clinical success was defined as a patient having technical success and could ambulate after four hours of bed rest without any complications related to hemostasis. Complications were classified according to the Society of Interventional Radiology guidelines (14). A major complication was any event that resulted in additional therapy, including an increased level of care, hospital stay beyond observation status, permanent adverse sequelae, and death. Included in the category of major complications is transfusion of blood products or additional interventions such as percutaneous drainage or endovascular procedures or surgery. All other complications were classified as minor.
Achieving hemostasis using the GH-150 was tolerable in all patients with minimal groin pain or discomfort despite the prolonged device application. No patients complained of any ischemic signs or symptoms during device application, although the ipsilateral dorsalis pedis was weakly palpable during the 10-minute high-volume (150 ml) application (Fig. 2). Technical success was achieved in 38 patients (95%). In two patients, persistent oozing of blood was noted despite additional inflation of the bulb; eventually, hemostasis was achieved by manual compression. Both the technical failures had an INR < 1.5 and a platelet count > 50,000/µl, and their body mass indices were 24.53 and 25.96, respectively. The arteriotomy sites of the technical failures were just above the inguinal crease, which were the routine arteriotomy sites at our institution. Additional inflation was not necessary in other patients.
Upon removal of the lightly-applied device after four hours, minimal oozing was noted in another patient, which was successfully controlled by the simple placement of a sand bag for 20 minutes. The remaining 37 patients were able to initiate ambulation according to the protocol without the need for any additional measures (clinical success, 93%). No patients experienced any late complications regarding hemostasis during an average follow-up period of 238 days (range, 26-407 days). Twenty six patients underwent a successful re-arteriotomy of the ipsilateral femoral artery for an average of 155 days (range, 24-377 days) after the initial use of the GH-150.
Previous reports on the use of pneumatic compression devices are relatively limited compared with other ACDs, and have been focused on use during coronary intervention procedures (10-13). Currently, two types of pneumatic compression devices are available (Femostop; RADI Medical System, Uppsala, Sweden and Safeguard; Datascope, Fairfield, NJ). The Safeguard is structurally similar to the GH-150, except that it has a smaller bulb and no supplementary tapes. The key difference between the GH-150 and the Safeguard is that the Safeguard is aimed to assist manual compression, not to substitute for manual compression as does the GH-150. The Femostop is used to substitute manual compression like the GH-150. The Femostop consists of a large plastic arch bar positioned over the groin and a belt positioned under the patient's hips to hold the plastic bar. Because the plastic arch and the belt are intended to be reused, a switch to a pressure dressing is necessary to maintain hemostasis and to assist in reducing hematoma formation when the patient returns to the ward (15). In contrast, the GH-150 is a single use device in its entirety and an additional pressure dressing is not necessary because it is being kept in place and exerts pressure to maintain hemostasis for the entire duration of the compression.
Successful hemostasis rates of ACDs are reportedly to be as high as 95% in experienced hands (1, 16). However, each and every ACD has its own learning curve depending on the complexity of device deployment. Failure rates have been reported to be approximately 15% during early experience (17-19). Though a direct comparison with previous studies may be difficult due to different patient selection, it is noteworthy that the clinical success rate of this study was 93% (37 of 40). The two cases of technical failure in the current study occurred during the early period and another clinical failure involved minimal oozing of blood which was easily controlled by placing a sand bag. The technical failures were likely to be a result of the lack of experience because their coagulation profiles were tolerable and they were not obese. Appropriate adjustment of the bulb position such that the bulb fits into the groin and exerts maximal pressure on the arteriotomy site may be of greater importance than additional inflation of the bulb.
Given that oncologic procedures may be performed repeatedly in a single patient, the issue of re-puncture and repeated use of ACDs in a femoral artery is important (1). However, little is known about the result of the repeated use of ACDs in a femoral artery, and that the repeated use of ACDs may result in the formation of granulomas around the femoral artery induced by foreign bodies incorporated in ACDs is a valid concern. Pneumatic compression devices appear to be appropriate for use when repeated punctures are expected, because there are no foreign bodies involved. In the current study, approximately two-thirds of patients underwent re-puncture of the ipsilateral femoral artery as early as 24 days after the use of the GH-150 and no abnormalities were found, which may disturb the re-puncture.
It seems intuitive that the use of any ACDs is associated with increased cost and another advantage of the GH-150 over collagen plug-type or suture-mediated ACDs may be its lower cost ($80 versus $150-200) (20). However, whether the utilization of ACDs is cost effective depends on a variety factors including the frequency of vascular complications, the impact of the use of ACDs for the complication rate, and the potential for ACDs to substantially alter post-procedure management patterns. A comprehensive cost-benefit analysis of the GH-150 vs. manual compression or the GH-150 versus other ACDs is needed to determine the cost-effectiveness of the device use.
One of the advantages that support the use of ACDs is that collagen plug-type or suture-mediated ACDs may permit early ambulation, even in fully anti-coagulated patients because these types of ACDs provide active approximation of the arteriotomy sites (16, 17, 19, 21). For this reason, pneumatic compression devices which do not provide active approximation are not recommended in the clinical setting of intensive anticoagulation due to prolonged compression times (13). The liver plays a central role in the maintenance of hemostasis. Liver diseases, such as viral hepatitis or liver cirrhosis, are associated with a variety of hemostatic abnormalities by disturbing the synthesis of proteins required for hemostasis and affecting platelet count and function (22). In the current study, a substantial portion (16 of 40) of the patients had prolonged prothrombin times and/or necessitated transfusions of platelet concentrates due to underlying liver diseases. However, hemostasis was successfully achieved in all of these patients. Further studies are needed to verify the usefulness of the GH-150 in a therapeutic setting of anti-coagulation or in patients who have moderate or severe hemostatic abnormalities.
This study had a few limitations. First, the study was restricted to patients who underwent oncologic procedures. Therefore, our results cannot be directly extrapolated to coronary or peripheral interventions and further analyses are needed to fully assess the outcomes of subsets not included in the present study. Second, due to its retrospective design, the current study lacks ultrasound examinations to evaluate the complications associated with the arteriotomy and use of the GH-150. The prolonged application of the GH-150 might compromise the venous drainage, which may also be evaluated by ultrasound examinations. Third, although we applied the GH-150 with a set inflation volume and time to develop a standardized protocol for use, use of the GH-150 needs to be customized according to the clinical situation and patient characteristics in practice.
Our data suggest that the GH-150 is an easy-to-use device with a short learning curve which provides reliable hemostasis of the femoral arteriotomy sites in patients who undergo transarterial chemoembolization or bland embolization without other hemostasis measures.
Figures and Tables
Fig. 1
GH-150 applied to model doll. Supplementary tapes (arrows) are applied over wings of main body.

Acknowledgments
We thank our registered nursing and technical staffs who have undergone and monitored the hemostasis process.
References
1. Madigan JB, Ratnam LA, Belli AM. Arterial closure devices. A review. J Cardiovasc Surg (Torino). 2007. 48:607–624.
2. Bent CL, Kyriakides C, Matson M. Femoral artery stenosis following percutaneous closure using a starclose closure device. Cardiovasc Intervent Radiol. 2008. 31:814–816.
3. Brueck M, Bandorski D, Rauber K, Boening A. Percutaneous transluminal dilatation of inadvertent partial or complete occlusion of the femoral artery caused by Angio-Seal deployment for puncture site closure after cardiac catheterization. J Invasive Cardiol. 2010. 22:353–357.
4. Cooper CL, Miller A. Infectious complications related to the use of the angio-seal hemostatic puncture closure device. Catheter Cardiovasc Interv. 1999. 48:301–303.
5. Jang JJ, Kim M, Gray B, Bacharach JM, Olin JW. Claudication secondary to Perclose use after percutaneous procedures. Catheter Cardiovasc Interv. 2006. 67:687–695.
6. Kim YJ, Yoon HK, Ko GY, Shin JH, Sung KB. Percutaneous transluminal angioplasty of suture-mediated closure device-related femoral artery stenosis or occlusive disease. Br J Radiol. 2009. 82:486–490.
7. Stone PA, Campbell JE, Andrews KH, Bates MC. Posterior wall capture and resultant common femoral occlusion complicating StarClose access closure. J Vasc Surg. 2008. 48:469–471.
8. Dangas G, Mehran R, Kokolis S, Feldman D, Satler LF, Pichard AD, et al. Vascular complications after percutaneous coronary interventions following hemostasis with manual compression versus arteriotomy closure devices. J Am Coll Cardiol. 2001. 38:638–641.
9. Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004. 291:350–357.
10. Chamberlin JR, Lardi AB, McKeever LS, Wang MH, Ramadurai G, Grunenwald P, et al. Use of vascular sealing devices (VasoSeal and Perclose) versus assisted manual compression (Femostop) in transcatheter coronary interventions requiring abciximab (ReoPro). Catheter Cardiovasc Interv. 1999. 47:143–147. discussion 148.
11. Jaspers L, Benit E. Immediate sheath removal after PCI using a Femostop is feasible and safe. Results of a registry. Acta Cardiol. 2003. 58:535–537.
12. Juergens CP, Leung DY, Crozier JA, Wong AM, Robinson JT, Lo S, et al. Patient tolerance and resource utilization associated with an arterial closure versus an external compression device after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2004. 63:166–170.
13. Kunert M, Gremmler B, Schleiting H, Ulbricht LJ. Use of FemoStop system for arterial puncture site closure after coronary angioplasty. J Invasive Cardiol. 2004. 16:240–242.
14. Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009. 20:S425–S434.
15. Walker SB, Cleary S, Higgins M. Comparison of the FemoStop device and manual pressure in reducing groin puncture site complications following coronary angioplasty and coronary stent placement. Int J Nurs Pract. 2001. 7:366–375.
16. Khaghany K, Al-Ali F, Spigelmoyer T, Pimentel R, Wharton K. Efficacy and safety of the perclose closer s device after neurointerventional procedures: prospective study and literature review. AJNR Am J Neuroradiol. 2005. 26:1420–1424.
17. Assali AR, Sdringola S, Moustapha A, Ghani M, Salloum J, Schroth G, et al. Outcome of access site in patients treated with platelet glycoprotein IIb/IIIa inhibitors in the era of closure devices. Catheter Cardiovasc Interv. 2003. 58:1–5.
18. Warren BS, Warren SG, Miller SD. Predictors of complications and learning curve using the Angio-Seal closure device following interventional and diagnostic catheterization. Catheter Cardiovasc Interv. 1999. 48:162–166.
19. Balzer JO, Scheinert D, Diebold T, Haufe M, Vogl TJ, Biamino G. Postinterventional transcutaneous suture of femoral artery access sites in patients with peripheral arterial occlusive disease: a study of 930 patients. Catheter Cardiovasc Interv. 2001. 53:174–181.
20. Dauerman HL, Applegate RJ, Cohen DJ. Vascular closure devices: the second decade. J Am Coll Cardiol. 2007. 50:1617–1626.
21. Kim HY, Choo SW, Roh HG, Han H, Kim SS, Lee JY, et al. Efficacy of femoral vascular closure devices in patients treated with anticoagulant, abciximab or thrombolytics during percutaneous endovascular procedures. Korean J Radiol. 2006. 7:35–40.
22. Wada H, Usui M, Sakuragawa N. Hemostatic abnormalities and liver diseases. Semin Thromb Hemost. 2008. 34:772–778.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download