Abstract
Objective
The ventriculus terminalis (VT) in adults is a rare pathology. We report various MR imaging features of the adult VT.
Materials and Methods
Ten patients were included in this retrospective review.. All patients had undergone magnetic resonance (MR imaging with a surface coil that used two different 1.5T MR systems. All patients had undergone initial and follow-up MR imaging with contrast enhancement using gadopentate dimeglumine. Three patients underwent additional MR imaging using the echocardiogram-gated spatial modulation of magnetization (SPAMM) technique. If a shift in tagging band during the systolic phase was less than half of the band space, it was defined as a "non-pulsatile fluid". Two neuroradiologists independently reviewed these images, while clinical symptoms and outcomes were statistically analyzed between the treated and non-treated group.
Results
All cases presented an intramedullary cystic lesion in the conus medullaris and showed the same signal intensity as CSF. Three VTs had intracystic septation and cord edema, which were pathologically confirmed after surgery; two of these were associated with kyphotic deformity and spinal arteriovenous malformation. SPAMM-MRI of 3 patients demonstrated non-pulsatile fluid motion within the VT. In the treated group, clinical symptoms improved better than the non-treated group.
The ventriculus terminalis (VT) is a small ependymal-lined cavity within the conus medullaris, and is as a result of canalization and retrogressive differentiation during embryonic development. The VT has been described as a normal developmental phenomenon in newborns and pediatrics, but is a rare pathology in adults, with only 21 cases reported to date (1-4). The etiology and pathogenesis of this lesion remains unknown. Several hypotheses have been proposed for the development of VT. Some authors propose that cavitations might be associated with trauma, vascular disturbance, inflammatory, or compressive pathology of the spinal cord, and may interrupt communication between the VT and the central canal (5, 6).
Herein, we report various magnetic resonance (MR) imaging features of VT in 10 adults and correlate clinical and imaging data with the pathophysiology of the VT.
Ten patients (male : female = 7 : 3, median age = 46.5 years, ranging from 33 to 59 years) were enrolled from a retrospective review of medical data collected between January 2004 and March 2010. All patients had undergone initial and follow-up MR imaging with contrast enhancement using gadopentate dimeglumine (Gd-DTPA). Three patients had undergone additional MR imaging using the spatial modulation of magnetization (SPAMM) technique to evaluate cerebrospinal fluid (CSF) hydrodynamics of the VT. The following clinical data of all patients were reviewed: 1) the initial clinical symptom, 2) associated disease, and 3) changes of the clinical symptom during the follow-up period or postoperative results of the VT. Three patients were pathologically confirmed after operation and one patient was associated with spinal arteriovenous malformation (AVM). Six patients with the typical imaging of the VT were not treated, because there was no interval change in their clinical symptom during the follow-up period (more than 1-2 years) after their initial diagnosis by MR imaging. The institutional review board of our institution approved this retrospective study and the requirement for informed consent was waived.
Magnetic resonance imaging with a surface coil was performed using two 1.5T MR systems (Vision 1.5T and Avanto 1.5T; Siemens Medical Systems, Erlangen, Germany). All patients underwent T1-weighted and T2-weighted sagittal and gradient axial images and the parameters of the conventional T1- and T2-weighted sequences were optimized for each MR system. Additionally, contrast-enhanced T1-weighted sagittal and axial images of the lesion were obtained using Gd-DTPA (0.1 mmoL/kg body weight).
In three patients who underwent spatial modulation of magnetization (SPAMM)-MR imaging, a tagging radiofrequency pulse was applied after the trigger pulse, and a series of gradient-echo sequences followed. Electrocardiogram (ECG)-triggered multiphase images were obtained (repetition time/time to echo/diffusion time/flip angle = 43 milliseconds/7.2 milliseconds/0 to 600 milliseconds/20 degrees; matrix, 128 × 256; slice thickness, 6 mm; field of view, 250 mm; saturation thickness, 10 mm; and tagging band thickness, 2 mm). Sixteen phase-images at the same slice between every R-R wave of the ECG, as well as for each sagittal and coronal SPAMM-MR imaging were obtained. CSF hydrodynamics was analyzed according to shifts in the tagging band throughout the cardiac cycle upon SPAMM-MR imaging. The extent of the shift in the tagging band was calculated individually for CSF and intracystic fluid; measurements were taken at the point where the shift was at a maximum. If a shift in the tagging band was more than half of the space between the adjacent tagging bands, it was defined as a "pulsatile motion within the VT", but if a shift was less than half, it was defined as a "no pulsatile motion."
These images were reviewed independently by two experienced neuroradiologists and the following parameters were estimated based on the images: 1) size (maximal diameter), axial (central/eccentric) location and characteristics of the lesion (enhancement, septation, cord edema, concurrent disease), and 2) tagging band shifts within the lesion upon SPAMM-MRI. Clinical symptoms were divided into 3 categories: type 1, patients with nonspecific neurological symptoms or nonspecific complaints; type 2, presence of a focal neurological deficit (sensory or motor); and type 3, presence of sphincter disturbance (bladder or bowel symptom). The presence of a sphincter disturbance was correlated with several imaging findings, such as lesion size and location, then compared between the treated and the non-treated groups.
All of the VTs appeared to have cysts and the mean size of the lesions was 32.67 ± 18.5 mm, ranging from 15 to 68 mm. They were located in the center of the spinal cord and mainly in the lower thoracic region (T11/12). All cases were hypointense on T1-weighted imaging without enhancement and hyperintense relative to the spinal cord on T2-weighted imaging (Table 1). In 3 cases that used SPAMM-MRI, there was no movement of the tagging band within the cystic VT (Fig. 1). Three VTs had intracystic septations with perilesional edema (3/10, 30%), which were pathologically confirmed after laminectomy with myelotomy or cyst fenestration (Fig. 2). Three patients were associated with syringomyelia, spinal arteriovenous malformation (AVM), and kyphotic deformity, respectively.
According to the classification of clinical symptoms, type 1 symptoms were shown in 4 patients, type 2 symptoms in 3 patients and type 3 symptoms in 3 patients. Six patients, who had undergone conservative treatment, were presented as stable without progression of clinical symptoms during the follow-up period (median 13.5 months, ranging from 12 to 60 months). Although clinical symptoms had not improved markedly in the non-treated group, 3 patients showed improved clinical symptoms rapidly and one remained stable with no remarkable change after surgical or endovascular procedures. Moreover, it is likely that the degree of clinical symptoms will be correlated with the size of the conus lesion; the larger the size of conus lesion, the greater the severity of the clinical symptoms.
In an initial report in 1859 by Stilling, the VT was identified as a ventricular structure enclosed by ependymal tissue and was called a terminal ventricle, an ependymal cyst, or the fifth ventricle. In the 7th week after conception, the VT is detectable in the caudal neural tube, regression, and regressive differentiation begins on day 48. Though the process of canalization and retrogressive differentiation may normally activate, this ependymal structure is often present in the conus medullaris, which becomes identifiable in neonates and children upon ultrasonography or MR imaging (1, 7-9). In a postmortem study by Kernohan et al. (10) the VT was reported to be a "true ventricle" and communicated with neither the subarachnoid space nor the central canal of the spinal cord. Coleman et al. (3) reported that the dilated VT was found in 2.6% of pediatric patients (less than 5 years) without related symptoms. However, cystic dilatation of the VT in children may be associated with congenital anomalies, such as tethered cord syndrome, Chiari type I malformation, lipomyelomeningoceles, and lumbosacral lipoma (11-13).
Infrequently, the isolated dilatation of the VT is detected in the elderly, combined with clinical symptoms. Nassar et al. (6) remarked that intramedullary cystic lesion of the conus medullaris may be produced by trauma, hemorrhage, compressive pathology, or vascular impairment. The maximal diameter of the cystic VT in adults is reported to be larger than that of the pediatric VT, which concurred with our results. Of note, one patient was associated with kyphotic deformity, and cord edema was noted (patient 6) (Fig. 2). These imaging findings may support that progression of the adult VT may be associated with spinal canal narrowing and vertebral deformity. Furthermore, the VT of one patient was related to spinal AVM and the dilated VT had regressed 11 months after embolization (patient 9). Recently, Srivatanakul et al described that venous hypertension in the spinal cord induces the development of syringomyelia, which resolved after embolization in 3 cases (14).
Sigal et al. (5) described another hypothesis of the adult VT, which results from a lack of communication between the VT and the central canal. On this basis, CSF hydrodynamics of the adult VT was evaluated using specific MR imaging techniques. Recently ECG-gated SPAMM-MR imaging has been utilized to assess detailed motion and direction of CSF within the spinal cord (15). Wayte and Redpath (16) reported visualization of pulsatile CSF by showing shift in tagging bands on the cardiac-gated cine images of SPAMM. In support, a few authors reported the evaluation of CSF hydrodynamics using the SPAMM technique in syringomyelia, cervical stenosis and Chiari malformation, concluding that SPAMM-MR imaging was useful in the quantification of spinal CSF flow (15, 17-19). In these reports, the presence of pulsatile motion in the syrinx represented communication with CSF space and the syrinx, as well as the redundant wall capacity of the spinal cord. In our study, SPAMM-MR imaging was performed in only 3 patients, which showed no shifts in the tagging band to the adjacent bands within the VT. According to these findings, we inferred that there was "no pulsatile motion within the cystic VT," supporting the previous theory as additional evidence.
The characteristic feature of the VT in MR imaging is a cystic lesion of the distal central spinal cord canal without cord signal abnormality. However, in 3 patients who underwent surgical treatment (3/22, 13.6%) (Table 2), these lesions showed septations with cord edema, which were proven pathologically as an ependymal lining (Fig. 3). These unusual MR findings of the adult VT have not been reported yet in the literature and the cause of these findings are still unclear, although compressive myelopathy and kyphosis may be associated with cord edema. It is very important to consider a differential diagnosis of spinal neoplasm if the cystic lesion of the conus medullaris has septation and edema, although our cases were confirmed pathologically as the VT after surgical resection. Seo et al. (20) recently reported on a non-enhancing intramedullary cystic lesion of the conus, which was pathologically confirmed as spinal astrocytoma and was not to be disregarded in the differential diagnosis of non-enhancing intramedullary lesions.
The management of t adult VT is controversial, as some prefer non-operative management with serial imaging, while others favor surgical maneuvers for relief of neurological symptoms (4, 21-23). In our report, surgical or endovascular procedures were performed in 4 patients with progressing neurological symptoms. After the procedure, 3 patients showed improved neurologic symptoms and back pain was stabilized in one. Most cases in previous reports had been treated with surgical maneuvers (89%, 16/18) and demonstrated improvement of their clinical symptoms, similar to our results (Table 2). In review of the literature, a few authors reported on the postoperative clinical outcomes of the VT, which were evaluated according to their clinical presentation; patients with nonspecific neurologic symptoms were managed conservatively, whereas patients with focal neurological deficits or sphincter disturbance underwent surgical menagement (4).
There are some limitations to this retrospective study. Although the VT is a rare pathology, a small number of cases limit the interpretation of the results, such as correlation of clinical symptoms with the imaging findings, comparison between both groups, by using the statistical analysis. Second, only 3 patients had undergone additional SPAMM-MR imaging, which is not enough to be conclusive for the CSF hydrodynamics of the VT. Third, the median follow-up period was no longer than 14 months, despite the typical MR findings of the adult VT in 6 patients who underwent conservative treatment.
Although the adult VT is a rare pathology with typical imaging findings, it shows some unusual imaging features, such as septation and coexistence of spinal AVM, which may present clinical evidence for the pathophysiology of the adult VT. In addition, surgical maneuvers may be considered as a treatment option in case of the VT with progressive neurological symptoms.
Figures and Tables
Fig. 1
43-year-old man presented with back pain.
A. High signal-intensity T2-weighted sagittal image shows intramedullary cystic lesion in conus medullaris with. B. On spatial modulation of magnetization magnetic resonance sagittal image, there is no movement of tagging band in cystic ventriculus terminalis with cardiac pulsation (arrow).
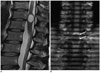
Fig. 2
50-year old man with left sciatic pain, who had kyphotic deformity. T2-weighted sagittal image shows cystic lesion of conus medullaris and accompanying cord edema (arrow) with thoracic kyphosis.
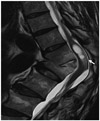
Fig. 3
T2-weighted coronal image shows cystic lesion of 33-year old man with urinary difficulty. Cystic lesion of conus medullaris has elements of septation and enlargement.
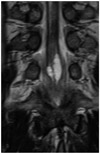
Table 2
Summary of Literature on Ventriculus Terminalis in Adults

Note.- m F/U = mean follow-up period, Tx = treatment, MR = magnetic resonance, cDisease = concurrent disease, mo = month, CM = Chiari Malformation, SD = spinal dysraphism, T = treated, NT = non-treated, NC = not confirmed, NF = neurofibromatisis, C = confirmed, NA = not available, AVM = arteriovenous malformation
References
1. Unsinn KM, Mader R, Gassner I, Kreczy A, Freund MC. Sonography of the ventriculus terminalis in newborns. AJNR Am J Neuroradiol. 1996. 17:1003–1004.
2. Kriss VM, Kriss TC, Babcock DS. The ventriculus terminalis of the spinal cord in the neonate: a normal variant on sonography. AJR Am J Roentgenol. 1995. 165:1491–1493.
3. Coleman LT, Zimmerman RA, Rorke LB. Ventriculus terminalis of the conus medullaris: MR findings in children. AJNR Am J Neuroradiol. 1995. 16:1421–1426.
4. de Moura Batista L, Acioly MA, Carvalho CH, Ebner FH, Tatagiba M. Cystic lesion of the ventriculus terminalis: proposal for a new clinical classification. J Neurosurg Spine. 2008. 8:163–168.
5. Sigal R, Denys A, Halimi P, Shapeero L, Doyon D, Boudghène F. Ventriculus terminalis of the conus medullaris: MR imaging in four patients with congenital dilatation. AJNR Am J Neuroradiol. 1991. 12:733–737.
6. Nassar SI, Correll JW, Housepian EM. Intramedullary cystic lesions of the conus medullaris. J Neurol Neurosurg Psychiatry. 1968. 31:106–109.
7. Unsinn KM, Mader R, Gassner I, Kreczy A. Ventriculus terminalis of the spinal cord in the neonate: a normal variant on sonography. AJR Am J Roentgenol. 1996. 167:1341.
8. Truong BC, Shaw DW, Winters WD. Dilation of the ventriculus terminalis: sonographic findings. J Ultrasound Med. 1998. 17:713–715.
9. Kriss VM, Kriss TC, Coleman RC. Sonographic appearance of the ventriculus terminalis cyst in the neonatal spinal cord. J Ultrasound Med. 2000. 19:207–209.
10. Kernohan JW. The ventriculus terminalis: its growth and development. J Comp Neurol. 1924. 38:107–125.
11. Byrd SE, Harvey C, Darling CF. MR of terminal myelocystoceles. Eur J Radiol. 1995. 20:215–220.
12. Taviere V, Brunelle F, Baraton J, Temam M, Pierre-Kahn A, Lallemand D. MRI study of lumbosacral lipoma in children. Pediatr Radiol. 1989. 19:316–320.
13. Erkan K, Unal F, Kiris T. Terminal syringomyelia in association with the tethered cord syndrome. Neurosurgery. 1999. 45:1351–1359. discussion 1359-1360.
14. Srivatanakul K, Songsaeng D, Ozanne A, Toulgoat F, Lasjaunias P. Spinal arteriovenous malformation associated with syringomyelia. J Neurosurg Spine. 2009. 10:436–442.
15. Lee KH, Chung TS, Jeon TJ, Kim YH, Chien D, Laub G. Application of spatial modulation of magnetization to cervical spinal stenosis for evaluation of the hydrodynamic changes occurring in cerebrospinal fluid. Korean J Radiol. 2000. 1:11–18.
16. Wayte SC, Redpath TW. Cine magnetic resonance imaging of pulsatile cerebrospinal fluid flow using CSPAMM. Br J Radiol. 1994. 67:1088–1095.
17. Park CH, Chung TS, Kim DJ, Suh SH, Chung WS, Cho YE. Evaluation of intrasyrinx fluid motion by spatial modulation of magnetization-magnetic resonance imaging in syringomyelia with long-term follow-up: a predictor of postoperative prognosis? J Comput Assist Tomogr. 2008. 32:135–140.
18. Sakas DE, Korfias SI, Wayte SC, Beale DJ, Papapetrou KP, Stranjalis GS, et al. Chiari malformation: CSF flow dynamics in the craniocervical junction and syrinx. Acta Neurochir (Wien). 2005. 147:1223–1233.
19. Lee SK, Chung TS, Kim YS. Evaluation of CSF motion in syringomyelia with spatial modulation of magnetization (SPAMM). Yonsei Med J. 2002. 43:37–42.
20. Seo HS, Kim JH, Lee DH, Lee YH, Suh SI, Kim SY, et al. Nonenhancing intramedullary astrocytomas and other MR imaging features: a retrospective study and systematic review. AJNR Am J Neuroradiol. 2010. 31:498–503.
21. Brisman JL, Li M, Hamilton D, Mayberg MR, Newell DW. Cystic dilation of the conus ventriculus terminalis presenting as an acute cauda equina syndrome relieved by decompression and cyst drainage: case report. Neurosurgery. 2006. 58:E585. discussion E585.
22. Dullerud R, Server A, Berg-Johnsen J. MR imaging of ventriculus terminalis of the conus medullaris. A report of two operated patients and a review of the literature. Acta Radiol. 2003. 44:444–446.
23. Celli P, D'Andrea G, Trillò G, Roperto R, Acqui M, Ferrante L. Cyst of the medullary conus: malformative persistence of terminal ventricle or compressive dilatation? Neurosurg Rev. 2002. 25:103–106.
24. Stewart DH Jr, King RB, Lourie H. Surgical drainage of cyst of the conus medullaris. Report of three cases. J Neurosurg. 1970. 33:106–110.
25. Robertson DP, Kirkpatrick JB, Harper RL, Mawad ME. Spinal intramedullary ependymal cyst. Report of three cases. J Neurosurg. 1991. 75:312–316.
26. Agrillo U, Tirendi MN, Nardi PV. Symptomatic cystic dilatation of V ventricle: case report and review of the literature. Eur Spine J. 1997. 6:281–283.
27. Matsubayashi R, Uchino A, Kato A, Kudo S, Sakai S, Murata S. Cystic dilatation of ventriculus terminalis in adults: MRI. Neuroradiology. 1998. 40:45–47.
28. Liccardo G, Ruggeri F, De Cerchio L, Floris R, Lunardi P. Fifth ventricle: an unusual cystic lesion of the conus medullaris. Spinal Cord. 2005. 43:381–384.
29. Ciappetta P, D'urso PI, Luzzi S, Ingravallo G, Cimmino A, Resta L. Cystic dilation of the ventriculus terminalis in adults. J Neurosurg Spine. 2008. 8:92–99.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download