Abstract
Objective
Device- or technique-related air embolism is a drawback of various neuro-endovascular procedures. Detachable aneurysm embolization coils can be sources of such air bubbles. We therefore assessed the formation of air bubbles during in vitro delivery of various detachable coils.
Materials and Methods
A closed circuit simulating a typical endovascular coiling procedure was primed with saline solution degassed by a sonification device. Thirty commercially available detachable coils (7 Axium, 4 GDCs, 5 MicroPlex, 7 Target, and 7 Trufill coils) were tested by using the standard coil flushing and delivery techniques suggested by each manufacturer. The emergence of any air bubbles was monitored with a digital microscope and the images were captured to measure total volumes of air bubbles during coil insertion and detachment and after coil pusher removal.
Although the clinical consequences of small air bubbles introduced during endovascular procedures for cerebrovascular lesions are not yet well understood (1-7), the occurrence of any air embolism may be hazardous. Even catheter flushing with saline can result in air embolisms (8). Air embolism during cerebral angiography has also been observed using transcranial Doppler (9). Modification of the flushing technique and the use of heparin and air filters may, however, reduce the occurrence of these embolic phenomena (8, 10). Air-free endovascular procedures, however, require a deliberate effort to minimize the introduction of air bubbles, starting with the use of air-proof devices.
It is surprising that diffusion-weighted images obtained after embolization of unruptured intracranial aneurysms show the same or a more severe degree of microembolic lesions as after carotid stenting procedures, the latter of which are associated with a much higher risk of thromboembolism due to the nature of the procedure (11-14). To explain this, we moved our focus from thromboembolism to air embolism. We therefore assessed the air-proof qualities of the detachable embolization coils we use for the endovascular treatment of cerebral aneurysms. We designed an in vitro system to monitor the emergence of air bubbles during detachable coil delivery.
We constructed a closed circuit, made of transparent silicone tubes 4 mm in diameter and filled with normal saline, to simulate a patient's internal carotid artery (Fig. 1). A peristaltic pump (Ecoline VC-280; Ismatec, Wertheim-Mondfeld, Germany) was used for the pulsatility of the circuit. Air bubbles were removed from the circuit by continuous flushing of the tube with degassed normal saline at room temperature. The saline was degassed by sonification (Sonoswiss SW1; Ramsen, Switzerland) for 20 minutes. A digital microscope (Dino-lite; AnMo Electronics Corp, Taipei, Taiwan) was placed in a working segment of the silicone tube to monitor the emergence of any air bubbles. A ruler with a millimeter (mm) scale was placed within the magnified field adjacent to the silicone tube as an internal reference for the measurement of air bubble diameters.
A 6-Fr guiding catheter (Envoy; Codman, Raynham, MA, USA) was introduced into the working segment of the silicone tube through a rotating hemostatic valve, which behaved like a femoral artery access sheath, followed by a microcatheter (Excelsior 10 or 1018; Boston Scientific Corp., Fremont, CA, USA). Both the guiding catheter and the microcatheter were continuously flushed with pressurized (300 mm Hg), heparin-mixed (1000 unit/L) normal saline. No degassing procedure was performed for the flushing saline since this is not part of our real practice. The distal tip of the microcatheter was continuously monitored by the digital microscope. Detachable coils were prepared according to the instructions provided by each manufacturer.
We tested a total of 30 commercially available detachable embolization coil systems, including 7 Axium (Axium; EV3, Irvine, CA, USA), 4 Guglielmi (GDC 10; Stryker, Fremont, CA, USA), 5 MicroPlex (MicroPlex; Microvention, Tustin, CA, USA), 7 Target (Target; Stryker, Fremont, CA, USA), and 7 Trufill (Trufill Orbits; Codman, Raynham, MA, USA) coil systems. All the testing was done in four different sessions. We could not match the number of each coil system due to the limited availability of coils for testing. However, to minimize any possible differences in the test set up in four different sessions, we tested different types of coils in each session. Micrus coils could not be included due to local unavailability at that time.
By inserting the delivery sheath into the rotating hemostatic valve of the microcatheter, backward saline flushing could be achieved before insertion of the coil delivery system into the microcatheter. Profuse back flushing of the coil delivery system was done to eliminate preexisting air within the lumen of the delivery sheath. After detachment, the coil pushers were removed slowly and gently to avoid any possible negative pressure generation within the microcatheter lumen.
The tip of the microcatheter was monitored continuously from the beginning of the coil introduction to the removal of the coil pusher until the entire inner contents of the microcatheter lumen were flushed out. Images were captured with image-processing software (Dino Capture ver. 2; AnMo Electronics Corp. Taipei, Taiwan) at three representative phases of coil delivery: 1) during coil insertion, 2) during detachment, and 3) after removal of the coil pusher. The emergence of air bubbles and their total volume was monitored during the three phases. To measure the air bubble volume, the diameter of each bubble was measured and its spherical volume was calculated before summing up the volume of all observed bubbles. The Kruskal-Wallis test was used to compare the total air bubble volume for each coil (SPSS ver 11, Chicago, IL, USA).
Air bubbles emerged in 23 of the 30 tested coils (76.7%), most frequently right after the removal of the pusher wire. The total volume of air ranged from 0 to 23.42 mm3 (median: 0.16 mm3) (Table 1). A significantly greater amount of air emerged during the manipulation of Target detachment coils than during that of any other coils (p = 0.0004) (Figs. 2, 3, 4, and supplemented online video clip).
In general, so-called 'cerebral air embolism' is a serious clinical hazard, causing cerebral arterial occlusion and leading to increased intracranial pressure, cerebral infarction, and/or changes in the blood-brain barrier (BBB) (15-18). Several animal studies have shown that 0.5 mL/kg of intracarotid air was sufficient to cause permanent brain damage (19-21). It has been reported that the degree and type of brain injury are largely dependent on the size of these air bubbles (22). A microbubble may cause focal infarction if it is large enough to occlude the arteriole. Although rapid clearance of embolized air bubbles was observed in an animal study (23), smaller bubbles that passed through small arterioles could irritate their endothelium, causing transient BBB breakdown (15, 16). In rabbits, the intracarotid injection of 25 microliters (µL) of air bubbles resulted in marked dilatation of the affected pial arterioles, which persisted for 90 minutes and was directly associated with decreased regional blood flow and depression of neuronal function (15).
Fortunately, the volume of air we could observe with a single detachment coil system in our experiment was much lower than the amount of air found in those serious situations mentioned above. The volume varied according to coil type, with most bubbles having volumes smaller than 25 microliters. However, these bubbles cannot be ignored since, in most patients, multiple coils are implanted, retrieved and reinserted during endovascular treatment of cerebral aneurysms even in a typical procedure.
Every manufacturer recommends profuse back flushing of the coil delivery sheath before inserting the coils into the microcatheter, in order to remove any air bubbles in the sheath lumen. Even more, the Target coil system requires profuse flushing of the dispenser coil tube before the introducer sheath can be removed from the dispenser. As described earlier, we strictly followed the instructions provided by each manufacturer. Then what could be the possible mechanism of air bubble formation during and after delivery of the coil through the microcatheter? What could be the source of these air bubbles?
As Han et al. (24) already observed, the electrolytic mechanism of GDCs could be one of the sources of air bubble formation, despite their small volume. According to our experiment, however, most of the air bubbles were likely due to air introduced by the coil pusher, which was probably why most of the air bubbles were observed immediately after the removal of the coil pusher. It is of note that the volume of air bubbles from the Target coil system was significantly greater than that from any other coil system. We paid particular attention to the recently modified feature of the Target coil system, its pusher wire. The manufacturer modified the prototype GDC coil pusher to provide extra softness and flexibility of the distal segment of the pusher. They replaced approximately 45 cm of the distal segment of the prototype GDC coil pusher with a very flexible wound wire type pusher, which has a serrated irregular surface due to a wire wound around the primary pusher wire. We assume that the change of the surface characteristics could be the source of air bubble introduction. With this we can explain why the total amount of air volume between GDC coils and Target coils is significantly different. The air volume generated during electrolytic detachment was the same for both GDC coils and Target coils, while the volumes observed after removal of the coil pusher differed significantly. Successive tests should be carried out to verify our speculation. Furthermore, modifications by the manufacturer of the design and pusher material are required to resolve this potentially dangerous problem.
The major limitation of our in vitro experiment was the relatively low pressure of our circuit, which was approximately 30-40 mm Hg. This may exaggerate the total air volume due to the inverse relationship between air volume and applied pressure. This test should therefore be repeated under higher physiologic pressure, 80-120 mm Hg, which can be achieved by raising the height of the saline reservoir bottle. However, we monitored air bubbles at room temperature, which is lower than body temperature; this may have compensated for the relatively low pressure we used.
Although the volume of air bubbles differed depending on the coil types, we could observe a variable amount of air bubbles during the delivery of the detachable embolization coils. Air bubbles emerged most frequently after the removal of the coil pusher, especially with the Target coil systems.
Figures and Tables
Fig. 1
Closed circuit simulating patient's circulation and catheterization systems. (a) Pressure gauge, (b) silicone tube, (c) reservoir chamber, (d) peristaltic pump, (e) digital microscope and magnified view with small ruler for measurement, (f) guiding catheter, (g) microcatheter, (h) detachable coil and coil delivery system, and (i) pressurized saline bags.
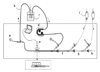
Fig. 2
Pictures captured by digital microscope during delivery of MicroPlex coil (Hydrosoft 6 mm, 8 cm).
A. Immediately after insertion of coil part. B. After detachment. C. Emergence of small air bubble (arrow) while removing pusher

Fig. 3
Pictures of air bubbles captured by digital microscope during delivery of Target coil system (2 mm, 4 cm).
A. Air bubble is noted right after completion of coil insertion. B. Several small air bubbles are noted while detaching coil. C. Numerous air bubbles are seen after removal of pusher.

References
1. Dunn GD. Microscopic air embolism and cerebral angiography. Lancet. 1993. 341:1215–1216.
2. Markus H. Transcranial Doppler detection of circulating cerebral emboli. A review. Stroke. 1993. 24:1246–1250.
3. Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999. 354:1594–1597.
4. Heiserman JE. Silent embolism after cerebral angiography--what harm? Lancet. 1999. 354:1577–1578.
5. Sayama T, Mitani M, Inamura T, Yagi H, Fukui M. Normal diffusion-weighted imaging in cerebral air embolism complicating angiography. Neuroradiology. 2000. 42:192–194.
6. Chuah KC, Stuckey SL, Berman IG. Silent embolism in diagnostic cerebral angiography: detection with diffusion-weighted imaging. Australas Radiol. 2004. 48:133–138.
7. Gupta R, Vora N, Thomas A, Crammond D, Roth R, Jovin T, et al. Symptomatic cerebral air embolism during neuro-angiographic procedures: incidence and problem avoidance. Neurocrit Care. 2007. 7:241–246.
8. Mamourian AC, Weglarz M, Dunn J, Cromwell LD, Saykin AJ. Injection of air bubbles during flushing of angiocatheters: an in vitro trial of conventional hardware and techniques. AJNR Am J Neuroradiol. 2001. 22:709–712.
9. Markus H, Loh A, Israel D, Buckenham T, Clifton A, Brown MM. Microscopic air embolism during cerebral angiography and strategies for its avoidance. Lancet. 1993. 341:784–787.
10. Bendszus M, Koltzenburg M, Bartsch AJ, Goldbrunner R, Günthner-Lengsfeld T, Weilbach FX, et al. Heparin and air filters reduce embolic events caused by intra-arterial cerebral angiography: a prospective, randomized trial. Circulation. 2004. 110:2210–2215.
11. Derdeyn CP. Diffusion-weighted imaging as a surrogate marker for stroke as a complication of cerebrovascular procedures and devices. AJNR Am J Neuroradiol. 2001. 22:1234–1235.
12. Soeda A, Sakai N, Murao K, Sakai H, Ihara K, Yamada N, et al. Thromboembolic events associated with Guglielmi detachable coil embolization with use of diffusion-weighted MR imaging. Part II. Detection of the microemboli proximal to cerebral aneurysm. AJNR Am J Neuroradiol. 2003. 24:2035–2038.
13. Kim SJ, Roh HG, Jeon P, Kim KH, Lee KH, Byun HS, et al. Cerebral ischemia detected with diffusion-weighted MR imaging after protected carotid artery stenting: comparison of distal balloon and filter device. Korean J Radiol. 2007. 8:276–285.
14. Chung SW, Baik SK, Kim Y, Park J. Thromboembolic events after coil embolization of cerebral aneurysms: prospective study with diffusion-weighted magnetic resonance imaging follow-up. J Korean Neurosurg Soc. 2008. 43:275–280.
15. Helps SC, Meyer-Witting M, Reilly PL, Gorman DF. Increasing doses of intracarotid air and cerebral blood flow in rabbits. Stroke. 1990. 21:1340–1345.
16. Helps SC, Parsons DW, Reilly PL, Gorman DF. The effect of gas emboli on rabbit cerebral blood flow. Stroke. 1990. 21:94–99.
17. Muth CM, Shank ES. Gas embolism. N Engl J Med. 2000. 342:476–482.
18. Um SJ, Lee SK, Yang DK, Son C, Kim KN, Lee KN, et al. Four cases of a cerebral air embolism complicating a percutaneous transthoracic needle biopsy. Korean J Radiol. 2009. 10:81–84.
19. Menzel M, Doppenberg EM, Zauner A, Soukup J, Reinert MM, Bullock R. Increased inspired oxygen concentration as a factor in improved brain tissue oxygenation and tissue lactate levels after severe human head injury. J Neurosurg. 1999. 91:1–10.
20. Goodman JC, Valadka AB, Gopinath SP, Uzura M, Robertson CS. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit Care Med. 1999. 27:1965–1973.
21. van Hulst RA, Lameris TW, Hasan D, Klein J, Lachmann B. Effects of cerebral air embolism on brain metabolism in pigs. Acta Neurol Scand. 2003. 108:118–124.
22. Hossmann KA. Experimental models for the investigation of brain ischemia. Cardiovasc Res. 1998. 39:106–120.
23. Annane D, Troché G, Delisle F, Devauchelle P, Hassine D, Paraire F, et al. Kinetics of elimination and acute consequences of cerebral air embolism. J Neuroimaging. 1995. 5:183–189.
24. Han MH, Kwon OK, Yoon CJ, Kwon BJ, Cha SH, Chang KH. Gas generation and clot formation during electrolytic detachment of Guglielmi detachable coils: in vitro observations and animal experiment. AJNR Am J Neuroradiol. 2003. 24:539–544.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download