Abstract
Sclerosing cholangitis in critically ill patients (SC-CIP) is a rare condition that is not familiar to many radiologists. In addition, the associated imaging findings have not been described in the radiological literature. We report a case of biliary cast formation with SC-CIP and describe the radiological findings of CT, magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiography (ERC). A diagnosis of SC-CIP should be considered in intensive care unit (ICU) patients with persistent cholestasis during or after a primary illness. The typical CT, MRCP and ERC findings include new biliary casts in the intrahepatic duct with multiple irregular strictures, dilatations, and relative sparing of the common bile duct.
Biliary cast formation almost always occurs in orthotopically transplanted livers as a consequence of complications. Biliary cast formation in non-transplanted livers is rare. However, in the past few years, gastroenterologists in Germany have observed and reported on an unusual sclerosing cholangitis in patients who have recovered from life-threatening disease in intensive care units (ICUs). This newly described entity has been named sclerosing cholangitis in critically ill patients (SC-CIP) (1-4). This new disorder is unfamiliar to many radiologists, and no reports focusing on the radiological findings associated with biliary cast formation in SC-CIP have been published. We report a case of biliary cast formation with SC-CIP and describe the computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiography (ERC) findings.
A 68-year-old man was transferred to our hospital for further evaluation of cryptogenic organizing pneumonia (COP). He had a six-week history of intermittent chills, myalgia, general weakness, and dyspnea. A contrast-enhanced chest CT acquired at a local medical center was reviewed by the chest radiologist at our hospital, whose first impression was also COP. Histologic confirmation was obtained by wedge resection via video-assisted thoracoscopic surgery (VATS). The patient received aggressive ICU treatment for COP with acute respiratory distress syndrome (ARDS), including positive end expiratory pressures (PEEP) mode mechanical ventilation. The patient did not have hypotension severe enough to require administering vasopressors, such as epinephrine or norepinephrine. Three weeks later, blood tests in the ICU revealed elevated alanine transaminase (ALT, 126 U/L) and alkaline phosphatase (ALP, 463 IU/L) levels but a normal total bilirubin level. The patient did not have any past medical history of hepatobiliary disease; therefore, the clinician suspected drug-induced hepatitis. The patient underwent abdominal ultrasound to exclude obstructive bile duct pathology or underlying chronic liver disease. An abdominal ultrasound revealed a slightly coarse parenchymal echotexture in the liver and a sludge ball in the gallbladder. There was no evidence of intrahepatic bile duct (IHD) or common bile duct (CBD) dilatation. Two weeks later, a contrast-enhanced abdominal CT was performed, due to increased ALP elevation, and a total bilirubin level of 4.39 mg/dL was observed. The contrast-enhanced abdominal CT showed suspicious multifocal strictures and irregularities in both IHDs and diffuse mild wall thickening with increased intraluminal attenuation in the right main IHD, which can be misinterpreted as a partial volume averaging effect (Fig. 1A, B). There was no evidence of thrombus formation or stenosis in the hepatic artery, portal vein, or hepatic vein. Our first impression was sclerosing cholangitis with bile sludge. We also considered Immunoglobulin G4-related sclerosing disease because combined interstitial pneumonia and sclerosing cholangitis can appear in that disorder. However, manifestation of IgG4-related sclerosing disease as sclerosing cholangitis without autoimmune pancreatitis is unusual, and IgG and IgG4 levels were normal. We reviewed the previous contrast-enhanced chest CT performed by the abdominal radiologist. There was no notable abnormality visualized in the hepatobiliary system on that CT. The presumed sclerosing cholangitis was newly developed during the hospitalization. MRCP was performed for further evaluation of the suspicious increased intraluminal attenuation in the right main IHD, which was needed for the ERC-assisted procedure by some possibility, and for the diagnosis of sclerosing cholangitis. The MRCP showed multiple strictures, irregularity, and a beaded appearance in both IHDs. There was an elongated intraluminal filling defect in both central IHDs that extended to the proximal CBD, which was suggestive of biliary casts in sclerosing cholangitis (Fig. 1C, D). The ERC revealed a diffuse, amorphous, elongated filling defect in both the central IHD and proximal CBD. A black-pigmented biliary cast was retrieved through the ampullar of Vater by basket (Fig. 1E). The patient was diagnosed as having biliary cast formation with SC-CIP. Further blood tests revealed improvements in the obstructive jaundice after the procedure, and the patient was discharged from the hospital after having received aggressive treatment including mechanical ventilation, for a total of six weeks.
One month later, a follow-up blood test administered on an outpatient basis again revealed an elevated total bilirubin level (8.60 mg/dL). The newly appeared biliary casts in both the IHDs and CBD were seen on MRCP, and the biliary casts were retrieved by basket on ERC. However, biliary casts were also noted from the central to the peripheral IHDs, and these casts could not be completely removed by basket (Fig. 1F). A contrast-enhanced abdominal CT performed 6 months later found clearer indications of multiple irregular strictures and dilatations in both IHDs (Fig. 1G, H). The patient is currently receiving ursodeoxycholic acid (UDCA) treatment.
Biliary cast formation almost always occurs in orthotopically transplanted livers and is associated with ischemic injury to the biliary epithelium. It occurs in 3% to 18% of orthotopic liver transplantations and results in biliary obstruction with infection and secondary hepatocyte and bile duct damage (5). The exact pathogenesis of cast development is unclear. It has been suggested that multiple etiological factors, such as biliary infection, bile duct damage, ischemia, fasting, and hemolysis, play roles in cast formation (5). Biliary cast formation in non-transplanted livers is rare, and there are a limited number of case reports of patients who had emergent cholecystectomy or antiphospholipid antibody syndrome (6). In our opinion, all of these previous cases may now be seen to have been SC-CIP, considering the clinical histories and imaging findings. SC-CIP is a recently described entity that occurs as a long-term complication in ICU patients during or after recovery from life-threatening illness. It is characterized by persistent, progressive cholestasis and findings of sclerosing cholangitis on cholangiography (1, 3, 4). The pathogenesis of SC-CIP is still under discussion. The most accepted pathogenesis involves arterial hypoperfusion of the perivascular plexus, and leads to ischemic injury of the bile duct epithelium, which is exclusively supplied by the perivascular plexus; biliary cast formation and subsequent biliary infection follow (1, 3, 7, 8). Accordingly, many SC-CIP cases require mechanical ventilation with high PEEP mode for severe respiratory problems or a high dose of vasoconstrictors, which can disturb the patient's hepatosplanchnic hemodynamics.
Clinical signs during the initial phases of SC-CIP are not specific and are similar to those of endotoxin-associated jaundice. SC-CIP usually begins with rapidly increasing serum levels of ALP and gamma-glutamyl transferase (GGT), as well as elevated serum bilirubin levels. However, the degree of bilirubin elevation is often less pronounced than that of ALP and GGT (1, 7). Despite apparent recovery from the primary illness, persistent cholestasis is observed, and this is the main clinical finding that discriminates SC-CIP from other types of cholestasis (7). Jaeger et al. (4) have reported that the initial cholestasis is noted at a median of 6.5 days (with a range of 4-11 days) after the initial injury, and Gelbmann et al. (1) have reported that elevated ALP and GGT levels are observed at a mean of 9.4 ± 6.1 days (with a range of 4-31 days) after the beginning of mechanical ventilation.
The histological characteristics of SC-CIP have been described by Gelbmann et al. (1), Scheppach et al. (2), and Benninger et al. (3). In the first 1-3 months after the initial cholestasis, the histological features of SC-CIP are quite variable and can range from mild, nonspecific changes to findings consistent with chronic bile duct obstruction. Within 4-12 months of the initial cholestasis, a histological analysis shows uniformly progressive fibrosis of the IHD with bile duct proliferation, portal inflammatory infiltrates, periductal fibrosis, bridging fibrosis, and canalicular cholestasis. After 12-24 months, incipient or overt liver cirrhosis is usually diagnosed.
The cross-sectional imaging findings for SC-CIP in a retrospective literature review are multiple irregular strictures, dilatations, a beaded appearance, and wall thickening of IHD, which are mimicking primary sclerosing cholangitis (PSC) (2). Ruemmele et al. (7) and Esposito et al. (10) have reported that the earliest event in SC-CIP may be extensive biliary cast formation with subsequent infections, and this is represented by intraductal filling defects in MRCP and ERC. Several weeks after the initial cholestasis, multiple irregular strictures or dilatations with biliary cast formations can often be observed. In addition, they reported that several months after the initial injury, advancing rarefaction of the small bile ducts and progressive sclerosis can often be observed. In our case, an increase in intraluminal attenuation in the diffusely thickened right main IHD was suspicious on the CT exam, and it was confirmed as biliary casts on ERC. However, biliary casts in the peripheral IHD or left main IHD were not detected in the contrast-enhanced CT exam. The ability to evaluate biliary casts from a CT exam may be limited. Irregular elongated filling defects on MRCP and ERC, which are representative of biliary casts, were noted in both IHDs and extended to the proximal CBD. Multiple irregular strictures, dilatations, and saccular ectasia were also seen in both IHDs. However, there was no evidence of CBD stenosis or irregularities. Jaeger et al. (4) have reported that multifocal strictures and beading are only seen in the IHDs without CBD involvement and that biliary casts formed in the IHDs can extend to the CBD. This finding in our study was consistent with the ERC and MRCP findings of other SC-CIP studies (2, 4, 6, 9). We believe that the SC-CIP imaging findings in the early stages are similar to those of typical sclerosing cholangitis, although biliary cast formation in the IHDs is not a typical finding in primary or other forms of secondary sclerosing cholangitis. In the later stages of SC-CIP, the cholangiography findings are similar to those of ischemic cholangitis and long-term PSC, which consisted of the destruction of the biliary tree rather than the usual sclerosing cholangitis (7, 8).
Radiologically, the differential diagnosis for biliary cast formation in SC-CIP includes ischemic cholangiopathy, PSC, and other forms of secondary sclerosing cholangitis. Ischemic cholangiopathy almost always occurs in transplanted livers as a consequence of complications and sometimes in other ischemic status, such as transarterial chemoembolization (TACE) complications or hepatic artery injury. However, it is still debatable whether SC-CIP is an ischemic-like cholangiopathy. Biliary cast formation is extremely rare in primary or other forms of secondary sclerosing cholangitis. In addition, most of the pathology in SC-CIP involves the IHD and spares the CBD. A clinical history that includes ICU hospitalization will be helpful for suggesting SC-CIP to a radiologist and for ruling out other causes of secondary sclerosing cholangitis.
With regard to the treatment of biliary casts in SC-CIP, endoscopic removal of as many as possible is recommended, and UDCA can also be used (6, 9). However, the progressive destruction of the biliary tree cannot be prevented; therefore, the only curative treatment option is liver transplantation. The prognosis for SC-CIP has not been established until recently. Gelbmann et al. (1) have reported that in the first two years after discharge from the hospital, eight of the 26 (30%) SC-CIP cases underwent liver transplantation or were waiting for liver transplantation, due to severe progressive cholestasis. Jaeger et al. (4) have reported that 45 months after the initial injury, one of the 10 (10%) SC-CIP cases underwent liver transplantation, and eight patients appeared to have slowly progressive disease. A study has reported that SC-CIP rapidly progresses to liver cirrhosis with a median survival of only 13 months if the patient does not undergo liver transplantation (7). Although establishing the prognosis of SC-CIP is still ongoing, the progression of SC-CIP is even more aggressive than that of other forms of secondary sclerosing cholangitis.
In conclusion, biliary cast formation in non-transplanted liver is rare. However, a diagnosis of SC-CIP should be considered in ICU patients with biliary cast formation and sclerosing cholangitis of the IHD in their CT, MRCP and ERC findings.
Figures and Tables
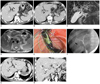 | Fig. 168-year-old man with biliary cast formation with sclerosing cholangitis in critically ill patients.
A. Contrast-enhanced axial computed tomography (CT) scan (5 mm slice thickness, portal phase) is suspicious of multifocal strictures and irregularities (arrows) in left intrahepatic duct (IHD). B. Contrast-enhanced axial CT scan (5 mm slice thickness, 3 min delayed phase) is suspicious of diffuse mild wall thickening (arrowheads) with increased intraluminal attenuation in right main IHD. C. 3D MIP magnetic resonance cholangiopencreatography image also reveals multiple strictures, irregularity, and beaded appearance in both IHDs. Elongated intraluminal filling defect, which indicates biliary cast (arrow) in proximal common bile duct (CBD) is seen. D. Heavily T2-weighted axial image shows intraductal filling defect, which is representative of biliary cast, in proximal CBD (arrow). E. Endoscopic removal of biliary cast after sphincterotomy. Endoscopic image shows black pigmented biliary cast that was retrieved through ampulla of Vater by basket. F. Seven weeks after initial cholestasis, ERC shows multifocal strictures, irregular dilatations in both IHD and biliary casts (arrow) in peripheral IHD. G, H. After 6 months, thin section contrast-enhanced axial CT scan (1.5 mm slice thickness, portal phase) and coronal scan (5 mm slice thickness) more clearly shows multiple irregular strictures and dilatations (arrowheads) in both IHD.
|
References
1. Gelbmann CM, Rümmele P, Wimmer M, Hofstädter F, Göhlmann B, Endlicher E, et al. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007. 102:1221–1229.
2. Scheppach W, Druge G, Wittenberg G, Mueller JG, Gassel AM, Gassel HJ, et al. Sclerosing cholangitis and liver cirrhosis after extrabiliary infections: report on three cases. Crit Care Med. 2001. 29:438–441.
3. Benninger J, Grobholz R, Oeztuerk Y, Antoni CH, Hahn EG, Singer MV, et al. Sclerosing cholangitis following severe trauma: description of a remarkable disease entity with emphasis on possible pathophysiologic mechanisms. World J Gastroenterol. 2005. 11:4199–4205.
4. Jaeger C, Mayer G, Henrich R, Gossner L, Rabenstein T, May A, et al. Secondary sclerosing cholangitis after long-term treatment in an intensive care unit: clinical presentation, endoscopic findings, treatment, and follow-up. Endoscopy. 2006. 38:730–734.
5. Parry SD, Muiesan P. Cholangiopathy and the biliary cast syndrome. Eur J Gastroenterol Hepatol. 2003. 15:341–343.
6. Gleeson FC, Czaja AJ, Baron TH. Successful endoscopic management of biliary cast syndrome in nonliver transplant patients. J Clin Gastroenterol. 2008. 42:752–755.
7. Ruemmele P, Hofstaedter F, Gelbmann CM. Secondary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol. 2009. 6:287–295.
8. Zimmer V, Raedle J, Treiber G, Lammert F. Biliary cast syndrome in sclerosing cholangitis. Dig Liver Dis. 2011. 43:e4.
9. Katsinelos P, Kountouras J, Chatzimavroudis G, Zavos C, Pilpilidis I, Paroutoglou G. Combined endoscopic and ursodeoxycholic acid treatment of biliary cast syndrome in a non-transplant patient. World J Gastroenterol. 2008. 14:5223–5225.
10. Esposito I, Kubisova A, Stiehl A, Kulaksiz H, Schirmacher P. Secondary sclerosing cholangitis after intensive care unit treatment: clues to the histopathological differential diagnosis. Virchows Arch. 2008. 453:339–345.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download