Abstract
Various anatomic anomalies have been considered the causes of nutcracker syndrome (NCS). Posterior NCS refers to the condition, in which vascular narrowing was secondary to the compression of the retroaortic left renal vein while it is crossing between the aorta and the vertebral column. Here, we report an unusual case of posterior NCS associated with a complicated malformation of the interrupted left inferior vena cava with azygos continuation and retroaortic right renal vein, diagnosed by both color Doppler ultrasonography and CT angiography.
Nutcracker syndrome is generally referred to as the compression of the left renal vein between the superior mesenteric artery and aorta (1). This compression leads to LRV hypertension and results in direct communication of the renal vein with the calyceal cavity and the varicosities of the collateral pathway of the LRV (2).
The pathophysiology of nutcraker syndrome (NCS) is not fully understood. Renal varicosities or abnormal branching of the superior mesenteric artery from the aorta are the most common factors considered responsible for this condition (1, 2). The other causes, such as compression of the retroaortic LRV between the aorta and spine (which is referred to as 'posterior NCS' in contrast to the more common 'anterior NCS') or aberrant right renal artery constricting the LRV, are uncommon (3, 4). However, three recently reported cases of nutcracker phenomenon associated with the left inferior vena cava (LIVC) suggest that some anomalies of the inferior vena cava (IVC) can also impair LRV blood outflow and result in NCS (4-6).
Here we reported a case of complicated malformation of LIVC with interrupted IVC and azygos continuation and retroaortic right renal vein causing posterior NCS, which has been diagnosed by both color Doppler ultrasound and computed tomographic angiography (CTA). To the best of our knowledge, this is the first report of an association between a complicated anomaly of the LIVC and NCS.
A 42-year-old female patient was referred to our hospital for renal ultrasonographic scanning. She had a past medical history of intermittent microscopic hematuria or clots for nearly 10 years' duration. These symptoms were exacerbated by a continuous mild dull lumbago for almost 3 months. Abdominal physical examination found no palpable mass and auscultation did not disclose any bruits. Laboratory studies showed 3+ hematuria. Urinary red blood cell count was 10-29 per high-power field. The morphology of urinary red cells by phase-contrast microscopy revealed 53% dysmorphic cells. Results of electrolyte and renal function tests were normal. Laboratory data showed no other positive findings. Informed consent was obtained before she entered the study.
Renal ultrasonography showed no anatomic defect on either side. However, oblique transverse scanning of the upper abdomen showed two tubular structures on both sides of the abdominal aorta above the level of the renal vein. Doppler ultrasound also revealed vascular signals at these tubular structures. We traced the vessels in an effort to scrutinize every segment of these two tubular structures and found that the right common iliac vein crossed the vertebra and formed the left-sided infrarenal IVC (L-IVC) along with the left common iliac vein. The right renal vein (RRV) traveled retroaortically and joined the L-IVC (Fig. 1A). The post-anterior diameter of the hilar portion and that of the retroaortic stenotic portion of the RRV were 8.8 mm and 2.0 mm, respectively, revealed by grey-scale ultrasound, which, with peak velocities, was demonstrated by a spectral Doppler of 26 cm/s and 87 cm/s, respectively. The diameter ratio and the peak velocity ratio between the dilated portion and the stenotic portion were 4.4 and 3.4, respectively. After collecting blood from the right and left common iliac veins and the renal veins, the L-IVC crossed the midline retroaortically and flowed into the right-sided suprarenal IVC (R-IVC) with turbulent, high-velocity flow (Fig. 1B, C). The diameters of the L-IVC at the level of the LRV and the dilated portion of the LRV near the hilus and the diameter of the IVC at the stenotic portion were 18.6 mm, 14.3 mm and 2.3 mm, respectively, with the peak velocities of 30 cm/s, 27 cm/s and 149 cm/s, respectively. Correspondingly, the diameter ratio between the dilated portion of LRV and the stenotic portion of L-IVC was 6.2, accompanied by the peak velocity ratio of 5.5. The hepatic veins failed in converging with the R-IVC (Fig. 1D). Below the level of the RRV, no tubular structure was found at the right lateral portion of the aorta.
The next day, computed tomographic venography scanning was performed for further evaluation. The results demonstrated that the RRV traveled retroaortically to the L-IVC. After collecting blood from the right and left common iliac veins and the renal veins, the L-IVC traversed retroaortically to join the R-IVC above the level of the celiac artery. The R-IVC ascended with the azygos instead of joining to the hepatic vein. In addition, paravertebral collateral veins in the right lumbar region traveled upward tortuously and drained into the R-IVC (Fig. 1E-G). The findings of both color Doppler ultrasound and CTA revealed that the anomaly of the IVC in our patient was LIVC with continuation of the azygos, existence of a retroaortic RRV, and absence of the posthepatic segment of the IVC (Fig. 1H).
Cystoscopy was performed to evaluate otherwise asymptomatic episodes of microscopic hematuria, which demonstrated no positive findings. The patient refused our suggestion to take renal venography for further evaluation.
By comprehensive analysis the data obtained by image findings, together with the patient's typical history and other laboratory findings, she was diagnosed as the posterior NCS associated with a complicated malformation of the LIVC.
The normal IVC is composed of four segments: hepatic, suprarenal, renal, and infrarenal. Formation of the IVC is extremely complex. Its evolutionary process involves the development, regression, and anastomosis of three pairs of fetal veins: the posterior cardinal veins, subcardinal veins, and supracardinal veins, in the embryonic life during the sixth to eighth week of gestation (7). The LIVC is the result of the persistence of the left supracardinal vein with regression of the right supracardinal vein. The prevalence of LIVC is 0.2% to 0.5% (7). Moreover, this case was more complicated because of the continuation with the azygos, the existence of the retroaortic RRV, and the absence of the posthepatic segment of the IVC. Consequently, the pathology may be accompanied by the atrophy of the right subcardinal vein, the persistence of the dorsal limb as well as the regression of the ventral limb of the right renal collar, and the failure to form the right subcardinal-hepatic anastomosis (7). Furthermore, interruption of the IVC with azygos continuation is often associated with congenital heart disease, polysplenia, and less commonly, asplenia. In our patient no congenital heart diseases or other anomalies were found, so we have reason to believe that this condition is extremely rare.
Diagnosis of the anomalies of IVC may have significant clinical implications, especially during retroperitoneal surgery or in the treatment of thromboembolic diseases (5-7). The majority of abnormal IVCs are clinically asymptomatic and only recognized incidentally during radiologic investigations performed for other reasons or during surgeries on the aorta or retroperitoneal structures or during postmortem examinations. In our literature review, we found only three cases of the anomalies of the IVC presenting as NCS. Ulusan et al. (5) first described data on an 80-year-old woman, who had a liver metastatic adenocarcinoma, and was incidentally found to have the anomaly of the LIVC. The authors believed that the compression of the LIVC between the aorta and superior mesenteric artery was responsible for her hematuria, a symptom of NCS. Since then, two other cases have been reported (4, 6). Regardless of the various causes of NCS, an outlet obstruction of the LRV is the outcome of NCS. Its typical symptoms, such as hematuria, left flank and abdominal pain, and varicoceles, are closely related to a backward venous renal hypertension due to the outflow impairment of the LRV and the development of compensating collateral circulation. In our present case, the aberrant vessels of the IVC resulted in two potential entrapments of the renal vein: 1) the pathway where the L-IVC drains into the R-IVC between the aorta and vertebra indirectly causing the hypertension of the LRV and 2) the location where the RRV runs to the L-IVC retroaortically. Theoretically, these abnormalities can result in the occurrence of NCS. The data obtained by sonography revealed that the ratios of the diameter and of the peak velocity in RRV between the dilated portion and the stenotic portion were 4.4 and 3.4, respectively, whereas those between the dilated segment of LRV and the narrowest segment of L-IVC were 6.2 and 5.5, respectively. CT angiography also demonstrated the existence of obvious dilatation of LRV, which we believed to be the main factor of the occurrence of the hematuria. In addition, the distension of the LRV and tortuously paravertebral collateral veins in the right lumbar region could explain the patient's feeling of a continuous dull lumbago for a long time.
Clinically, the diagnosis of NCS is based on the assessment of the pathologic anatomy and physiology (8). The newer imaging modalities such as CTA and magnetic resonance angiography have powerful abilities in providing accurate, three-dimensional reconstructive images in any desired plane and allowing better identification of these anomalies. Spectral Doppler ultrasound can give more details of the hemodynamics of the LRV at the site of compression. The case we presented here was first diagnosed by color Doppler ultrasound and then identified by CTA scanning with a high-quality delineated picture of the anomaly of the IVC. The dilated LRV as well as the L-IVC draining into the R-IVC in our patient were clearly visualized by CT and color Doppler ultrasound. Kim's study showed that the cutoff values for NCS as the ratios of both antero-posterior diameter and peak velocity between the distended and narrowed segments of the LRV are greater than 5, with sensitivity of 69%, 80% and specificity of 89%, 94%, respectively. But when combined with the cutoff value of more than 5 of the ratio of the antero-posterior diameter and the ratio of peak velocities divided by 2, the sensitivity and the specificity can be elevated to 90% and 100%, respectively (9). In our case, both the diameter ratio and the peak velocity ratio between the dilated segment of LRV and the narrowest segment of L-IVC were greater than 5 with a combined value of 5.9. Moreover, the morphology of urinary red cells is less than 75%, which is thought to be non-specific in nature (10). In review of our patient's typical history and clinical and radiologic findings, the diagnosis was most likely posterior NCS associated with complicated malformation of the LIVC.
In conclusion, a congenital anomaly of the IVC, although rare, may be one of the contributing factors for the development of NCS. With the aid of ultrasonography and the newer cross-sectional imaging modalities, variant venous anatomy should be identified and its clinical significance, which had not been mentioned in previous reports, should be evaluated thoroughly.
Figures and Tables
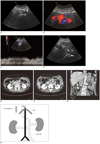 | Fig. 1Complicated malformation of left inferior vena cava presenting as nutcracker syndrome in 42-year-old woman.
Axial sonogram shows (A) right renal vein (RRV) (arrowhead) crossing retroaortically to area of left-sided infrarenal inferior vena cava (L-IVC) (long arrow) and no tubular structure parallel to aorta (short arrow) at right side, and (B) two tubular structures at left of aorta at level of left renal vein (LRV). (C) Spectral Doppler sonogram shows the narrowing portion of L-IVC (short arrow) with turbulent high-velocity blood flow between aorta and vertebral column. (D) Axial sonogram reveals that hepatic veins (arrowhead) do not flow to right-sided suprarenal inferior vena cava (R-IVC) (long arrow) but run to right atrium (RA). Ao = aorta
Axial CT angiogram is performed and demonstrates (E) right renal vein (RRV) (arrowhead) crossing retroaortically to left-sided infrarenal inferior vena cava (L-IVC) (long arrow) and (F) dilatation of both L-IVC (long arrow) and distal left renal vein (LRV) (short arrow), with the narrowing portion of RRV between aorta and lumber vertebral column. Tortuous paravertebral collateral veins can be seen in right lumbar area (arrowhead). (G) Coronal oblique image shows a dilated L-IVC receiving both LRV (short arrow) and RRV (arrowhead) and running to R-IVC retroaortically. The tortuously paravertebral collateral veins in right lumbar region are also seen (long arrow). (H) Schematic anatomy illustration shows multiple anomalies with the LIVC. Ao = aorta, R-IVC = right-sided suprarenal inferior vena cava
|
References
1. de Schepper A. ["Nutcracker" phenomenon of the renal vein and venous pathology of the left kidney]. J Belge Radiol. 1972. 55:507–511.
2. Beinart C, Sniderman KW, Saddekni S, Weiner M, Vaughan ED Jr, Sos TA. Left renal vein hypertension: a cause of occult hematuria. Radiology. 1982. 145:647–650.
3. Hartung O, Barthelemy P, Berdah SV, Alimi YS. Laparoscopy-assisted left ovarian vein transposition to treat one case of posterior nutcracker syndrome. Ann Vasc Surg. 2009. 23:413.e13–413.e16.
4. Fitoz S, Yalcinkaya F. Compression of left inferior vena cava: a form of nutcracker syndrome. J Clin Ultrasound. 2008. 36:101–104.
5. Ulusan S, Koc Z. Left inferior vena cava associated with nutcracker phenomenon. Firat Tip Dergisi. 2007. 12:151–153.
6. Gupta A, Naik N, Gulati GS. Mesoaortic entrapment of a left inferior vena cava. Indian J Radiol Imaging. 2010. 20:63–65.
7. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000. 20:639–652.
8. Wong HI, Chen MC, Wu CS, Fu KA, Lin CH, Weng MJ, et al. The usefulness of fast-spin-echo T2-weighted MR imaging in Nutcracker syndrome: a case report. Korean J Radiol. 2010. 11:373–377.
9. Kim SH, Cho SW, Kim HD, Chung JW, Park JH, Han MC. Nutcracker syndrome: diagnosis with Doppler US. Radiology. 1996. 198:93–97.
10. Pollock C, Liu PL, Györy AZ, Grigg R, Gallery ED, Caterson R, et al. Dysmorphism of urinary red blood cells--value in diagnosis. Kidney Int. 1989. 36:1045–1049.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download