Abstract
We report on a 41-year-old woman with a chest wall desmoid tumour who was successfully treated with a computed tomography (CT)-guided steroid injection. She presented with a palpable mass in the right upper chest wall and was treated by surgical excision and postoperative radiation therapy due to recurrence of the mass at the surgical site. At 20 months after the second operation, a recurrent mass was again detected in the anterosuperior portion of the previous surgical site on CT. We performed a CT-guided steroid injection weekly for 4 weeks by applying a mixture of 3 mL of triamcinolone acetonide (40 mg/mL) and 3 mL of 1% Lidocaine, administering 4-6 mL of the mixture, to the lesion. Six months later, CT showed a marked decrease in the size of the mass.
A desmoid tumour, also called aggressive fibromatosis, is a benign tumour originating from musculoaponeurotic structures throughout the body. The tumour can behave aggressively and infiltrate adjacent soft tissue structures or recur locally (1). Surgery, radiation therapy, and chemotherapy have been used to treat extra-abdominal desmoid tumours. However, their effectiveness is limited by frequent local recurrences (1, 2). This article describes our experience using a CT-guided steroid injection to treat a chest wall desmoid tumour. This is the first report of a CT-guided steroid injection for the treatment of a desmoid tumour.
A 41-year-old woman presented with a palpable mass in the right upper chest wall. A chest radiograph showed a round, soft-tissue density in the right upper hemithorax. Axial CT was obtained along with a 16-channel multi-detector CT (Sensation 16, Siemens Medical Solutions, Forchheim, Germany) with contrast enhancement. A round, 2.4 × 6-cm, isodense mass with enhancement surrounded the anterior arc of the right second rib. There was no evidence of cortical disruption, periosteal reaction, or bony erosion (Fig. 1A). The mass was excised and found to be an extra-abdominal fibromatosis.
One year later, she returned with right shoulder pain. Contrast-enhanced CT showed a 6 × 4-cm homogenously enhancing mass at the previous surgical site with cortical disruption at the anterior arc of the right first and second ribs (Fig. 1B). It was excised and the pathologic diagnosis was extra-abdominal fibromatosis (desmoid-type fibromatosis), with extension to adjacent skeletal muscle and bone. She was treated with postoperative radiation therapy, at a dose of 5000 cGY.
She underwent follow-up radiographs every three months in the thoracic surgery outpatient department. At 20 months after the second operation, an approximately 3.5 × 3.2 × 3.2-cm sized heterogeneously enhancing mass was detected in the anterosuperior portion of the previous surgical site on CT examination (Fig. 1C, D). A CT-guided biopsy revealed recurrent desmoid-type fibromatosis.
We performed weekly CT-guided steroid injections for four weeks. All injections were performed using a conventional spiral CT scanner (HiSpeed; GE Medical Systems, Milwaukee, WI, USA). First, the patient underwent imaging in the supine position with a section thickness of 5 mm with no contrast enhancement. Then, the skin was prepared in a sterile fashion, and 1% Lidocaine hydrochloride was administered with a 25-gauge hypodermic needle to anesthetise the skin and subcutaneous tissue. We used the coaxial technique with an 18-gauge needle and a 22gauge percutaneous ethanol injection therapy (PEIT) needle with multiple side holes (Hakko Medical, Nagano, Japan) to effectively inject the steroid. The mass was targeted using an 18-gauge needle using the coaxial technique, while the needle alignment was monitored by CT. The guide needle was anchored through the space between the right clavicle and anterior arc of the first rib. After anchoring, the axial CT was performed to confirm the adequacy of the position of the needle tip (Fig. 1E, F). We prepared a mixture of 3 mL of triamcinolone acetonide (40 mg/mL) and 3 mL of 1% Lidocaine. The mixture was injected using a 22gauge PEIT needle with multiple side holes, twice. The total volume injected was 4-6 mL. We repeated the CT-guided injection every week with the same dose of 46 mL of the mixture.
After three months, she underwent axial and coronal CT, but there was no interval change in the size of the recurrent mass. A CT examination 6 months later showed a marked decrease in the size of the mass (Fig. 1G, H), from 3.5 × 3.2 × 3.2 cm to 3.0 × 2.8 × 1.5 cm.
Desmoids also are known as aggressive fibromatosis. These tumours are histologically benign, but may behave aggressively at the local level, with multiple recurrences being common. In the management of desmoid tumours, treatment options include surgical resection, radiotherapy, anti-inflammatory agents, hormonal therapy, and chemotherapy. Wide excision is the treatment of choice for lesions that are relatively small and favourably located. However, the effectiveness of surgical resection is limited by the frequent local recurrence. Local recurrence rates after surgical excision are approximately 50% in patients older than 20 years (3). If a wide excision cannot be achieved without functional loss, radiation therapy is a treatment option. However, radiotherapy has a relapse rate of 31% for unresectable tumours (4).
Because of the infiltrative and recurring nature of the lesion in our patient, re-operation could not be the treatment of choice. Considering the presence of pain and continued rapid growth of the lesion, some type of intervention is suggested. Intralesional steroid injection is the most effective and widely used treatment for keloids. Triamcinolone acetonide is the most commonly used corticosteroid. Typically, 10 mg per linear centimetre of keloid is injected every 26 weeks. Triamcinolone reduces fibroblast proliferation and collagen synthesis, increases collagenase production, and reduces the levels of collagenase inhibitors as well as suppress inflammatory mediators and glycosaminoglycan synthesis (5, 6). On the basis of the knowledge that a desmoid tumor is composed of the proliferation of fibroblast and myofibroblast, as well as dense deposits of intracellular collagen fiber, we believe that a trial of intralesional corticosteroids could arrest the growth of desmoid tumor.
To our knowledge, this is the first report describing the treatment of recurrent desmoid tumours using a steroid injection.
Figures and Tables
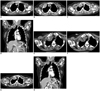 | Fig. 1Recurrent chest wall desmoid tumor in 41-year-old woman.
A. Contrast enhanced axial CT image shows round, 2.4 × 6-cm enhancing mass surrounded by anterior arc of right second rib. B. Contrast enhanced axial CT image shows 6 × 4-cm homogeneously enhancing mass at previous surgical site with cortical disruption at anterior arc of right first and second ribs. C, D. Contrast enhanced axial CT and coronal reconstruction images show 3.5 × 3.2 × 3.2-cm recurrent mass in anterosuperior portion of previous surgical site with heterogeneous enhancement. E, F. Axial CT image shows injection needle located in center of mass and CT image after injection. G, H. Follow-up chest axial CT and coronal reconstruction images after 6 months show marked decrease in size of mass.
|
References
1. Kujak JL, Liu PT, Johnson GB, Callstrom MR. Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skeletal Radiol. 2010. 39:175–182.
2. McDonald ES, Yi ES, Wenger DE. Best cases from the AFIP: extraabdominal desmoid-type fibromatosis. Radiographics. 2008. 28:901–906.
3. Dinauer PA, Brixey CJ, Moncur JT, Fanburg-Smith JC, Murphey MD. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics. 2007. 27:173–187.
4. Nuyttens JJ, Rust PF, Thomas CR Jr, Turrisi AT 3rd. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer. 2000. 88:1517–1523.
5. Al-Attar A, Mess S, Thomassen JM, Kauffman CL, Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006. 117:286–300.
6. Sherris DA, Larrabee WF Jr, Murakami CS. Management of scar contractures, hypertrophic scars, and keloids. Otolaryngol Clin North Am. 1995. 28:1057–1068.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download