Abstract
Objective
To retrospectively evaluate the frequency and risk factors for developing thrombus in a systemic vein such as the infrarenal inferior vena cava or the iliac vein, in which a balloon-occluded retrograde transvenous obliteration (B-RTO) catheter was indwelled.
Materials and Methods
Forty-nine patients who underwent B-RTO for gastric varices were included in this study. The B-RTO procedure was performed from the right femoral vein, and the B-RTO catheter was retained overnight in all patients. Pre- and post-procedural CT scans were retrospectively compared in order to evaluate the development of thrombus in the systemic vein in which the catheter was indwelled. Additionally, several variables were analyzed to assess risk factors for thrombus in a systemic vein.
Results
In all 49 patients (100%), B-RTO was technically successful, and in 46 patients (94%), complete thrombosis of the gastric varices was achieved. In 6 patients (12%), thrombus developed in the infrarenal inferior vena cava or the right common-external iliac vein. All thrombi lay longitudinally on the right side of the inferior vena cava or the right iliac vein. One of the aforementioned 6 patients required anticoagulation therapy. No symptoms suggestive of pulmonary embolism were observed. Prothrombin time-international normalized ratio and the addition of 5% ethanolamine oleate iopamidol, on the second day, were related to the development of thrombus.
Gastric varices are a complication of portal hypertension (1-3), though they are less common than esophageal varices (1). However, once gastric varices bleed, mortality rate without treatment is high: 45-55% (1, 4, 5). Thus, immediate management of bleeding gastric varices is necessary.
Balloon-occluded retrograde transvenous obliteration (B-RTO) is known to be an effective therapy for gastric varices caused by portal hypertension, if a dilated gastrorenal shunt exists (6-8). Disappearance or regression of gastric varices, after B-RTO, can be achieved at a high rate, ranging from 90-100%, according to previous reports (5-10). However, short-term complications of B-RTO have been reported (5, 7, 8, 10-16). Development of thrombosis in the left renal vein, main portal veins, superior mesenteric veins, and splenic veins are potential complications (11).
In daily clinical work, we experienced a case in which thrombosis developed after B-RTO in the infrarenal inferior vena cava and right common-external iliac vein into which the B-RTO catheter was inserted. The cause was presumed to be the indwelling catheter that had been left in place overnight, according to our procedure. To our knowledge, there have been no investigations into the development of thrombosis in a major systemic vein other than renal veins such as the infrarenal inferior vena cava and iliac vein. Therefore, we retrospectively evaluated the frequency and risk factors for the development of thrombus in the systemic vein, in which the catheter was indwelled with the exception of the left renal vein, in which a thrombus may extend from the gastrorenal shunt.
Between June 2004 and September 2010, 52 patients underwent B-RTO for gastric varices with a gastrorenal shunt which occurred after portal hypertension in institutions within our hospital system. In all patients, endoscopy revealed active bleeding (spurting or oozing) (n = 3) or showed signs of recent bleeding and/or growing gastric varices during the observation period (n = 49). We investigated data on the 49 patients (24 men and 25 women; mean age, 65 years; age range, 40-82 years) who underwent computed tomography (CT) after B-RTO. Causes of their liver cirrhosis were hepatitis B (n = 5), hepatitis C (n = 28), chronic alcohol ingestion (n = 12), and others (n = 4). According to the Child-Pugh classification, 23 patients were in class A, 22 in class B, and 4 in class C. The mean Child-Pugh score of the patient group was 6.8 ± 1.6. Of the 49 patients, 12 had concomitant hepatocellular carcinoma (HCC).
The procedure was performed by one of five interventional radiologists in our hospital system; these individuals had extensive experience with the B-RTO procedure. These institutions did not require institutional review board approval for this type of clinical observational study. Principles of the Declaration of Helsinki were followed, and informedconsent was obtained from all patients.
We performed B-RTO according to the method described by Kanagawa et al. (6). Briefly, after an 8-F curved catheter sheath-introducer (Medikit, Tokyo, Japan; Terumo Clinical Supply, Gifu, Japan) was inserted from the right femoral vein to the left renal vein, with the patient under local anesthesia, a 6.5-F balloon catheter with a balloon 22 mm in diameter (Artec Balloon catheter, Create Medics, Yokohama, Kanagawa, Japan) or a 6-F balloon catheter with a balloon 20 mm in diameter (Selecon balloon catheter, Terumo Clinical Supply, Gifu, Japan; Moiyan, Miyano Medical Instruments, Hyogo, Japan) was advanced to the left renal vein through the sheath-introducer. In 3 of the 49 cases, the 8-F sheath-introducer (Medikit) was inserted into the inferior vena cava, after which the balloon catheter was advanced from the inferior vena cava to the left renal vein. After insertion of the balloon catheter, the tip was wedged into the end of the gastrorenal shunt. The balloon was inflated in order to occlude the gastrorenal shunt. Retrograde venography was performed in order to identify gastric varices and their inflowing and outflowing vessels and to evaluate the degree of outflowing vessels other than the gastrorenal shunt.
If collateral veins other than the gastrorenal shunt were visualized, they were embolized with various combinations of microcoils, 50% glucose solution, and/or a small amount (5%) ethanolamine oleate iopamidol (EOI) (Grelan Pharmaceutical, Tokyo, Japan). Retrograde venography was performed in order to determine whether the entire varix was revealed and whether the outflowing vessels had disappeared or decreased. Then, the sclerosing agent, 50% glucose solution and/or 5% EOI, was slowly administered from the balloon catheter in order to fill the entire gastric varix while the balloon was inflated. The procedure was determined to be a technical success if the entire gastric varix was shown to be filled with the sclerosing agent on the fluoroscopic image. The entire setup was left in place overnight. If the retrograde venography performed the following morning indicated incomplete thrombosis of the varices, the sclerosing agent was added (n = 35). An incomplete thrombosis was declared even if only a tiny part of the gastric varix was stained densely. The sclerosing agent was left from 30 minutes to 4 hours, after which it was aspirated. The balloon was deflated and the balloon catheter was withdrawn. During these procedures, 4000 units of human haptoglobin were administered intravenously in order to prevent renal failure owing to hemolysis. Details of the B-RTO procedure have been described elsewhere (17).
A contrast-enhanced abdominal multi-detector row computed tomography (MDCT) was performed before B-RTO, as well as 5 to 15 days (mean 7.3 days) after B-RTO, to assess the thrombosis of the gastric varices and to determine the presence of complications, including a thrombus in a systemic vein. The MDCT unit used for this evaluation was either a 64-detector row CT (Brilliance 64, Philips Medical Systems, Cleveland, OH, USA) or a 16-detector row CT (Aquillion 16, Toshiba Medical Systems, Tokyo, Japan). Non-ionic iodinated contrast material was injected via an antecubital vein. The injection duration was set at 25 to 30 seconds, and doses of contrast material, tailored according to patients' body weights, were fixed at 500 to 600 mg I/kg. Scan acquisition for the first phase image was obtained with a 15-second delay after the automatic scan was triggered by contrast enhancement (170 HU) in the aorta at the bifurcation of the celiac artery. Scan acquisition for the second phase image was obtained with a 120- to 135-seconds delay after injection of contrast material was completed. Contiguous transaxial images, with 1 mm (n = 38) and/or 5 mm (n = 49) thickness without gaps, were reconstructed from the volumetric data set on each MDCT. In 37 of the 49 patients, the CT scans were obtained in a craniocaudal direction from the top of the liver to the level of the lower pole of the kidney. In the remaining 12 patients, CT scans were obtained to the level of the inferior border of the pubis. Images reconstructed with a width of 5 mm and, if necessary, with a width of 1 mm, were evaluated. For the present report, pre- and postprocedural CT scans were retrospectively compared by two radiologists who paid special attention to the development of thrombus in the systemic veins in which the catheter was indwelled; these veins were the infrarenal inferior vena cava, right iliac vein and/or right common femoral vein. In addition, the development of thrombus in the suprarenal inferior vena cava, the right renal vein, the left iliac vein and/or the left common femoral vein were evaluated. Thrombus in the left renal vein, in which a thrombus may extend from the gastrorenal shunt, was not evaluated. Decisions were reached through consensus between the two radiologists.
Issues investigated were 1) incidence of the development of thrombus in the systemic veins evaluated, 2) location of thrombosis in these veins, and 3) risk factors for thrombosis developing in these veins. The variables analyzed in order to determine risk factors for thrombosis in system veins were: age, sex, Child-Pugh score, presence of HCC, platelet count before B-RTO, prothrombin time-international normalized ratio (PT-INR) before B-RTO, grade of gastric varices according to Hirota et al. (8), amount of 5% EOI used, addition of 5% EOI on the second day, duration of indwelling of the catheter, tortuosity of the inferior vena cava, and the distance between the left adrenal vein and the junction of the left renal vein and the inferior vena cava. Tortuosity of the inferior vena cava, and distance between the left adrenal vein, and the junction of the left renal vein and the inferior vena cava were evaluated by multiplanar reconstruction images. Tortuosity was thought to be present if the inferior vena cava was angulated to more than 25 degrees on the coronal image of contrastenhanced abdominal MDCT.
For statistical analysis, commercial software (JMP 5.1; SAS Japan, Tokyo, Japan) was used. All variables were compared using the Fisher's exact test if the characteristics were qualitative and by the Wilcoxon rank-sum test if characteristics were quantitative.
A p value of less than 0.05 was considered to indicate a statistically significant difference for all analyses.
In all 49 patients (100%), B-RTO was technically successful, and in 46 of the 49 patients (93.9%), complete thrombosis of the varices was observed on the contrast-enhanced CT scans obtained 5 to 15 days after B-RTO. B-RTO was repeated in 1 of the 3 patients in whom thrombosis was incomplete. Reduction of the varices was seen in the remaining 2 patients on follow-up CT images and/or endoscopy. The total dose of 5% EOI injected ranged from 6-80 mL (mean dose, 32 mL). The mean dose of 5% EOI injected on the first day was 23 mL (range, 5-40 mL), and the mean dose of 5% EOI added on the second day was 9 mL (range, 0-40 mL).
In 6 of the 49 patients (12.2%), a thrombus developed in the infrarenal inferior vena cava and/or the right common-external iliac vein (Figs. 1, 2). In these patients, the thrombus was located in a systemic vein into which the B-RTO catheter had been inserted, while no thrombus was observed in the systemic veins into which the B-RTO catheter had not been inserted. One of the 6 patients with thrombus needed anticoagulation therapy with warfarin (Warfarin; Eizai, Tokyo, Japan) because the thrombus was relatively large and almost occlusive in the iliac vein. A decrease in the thrombus was confirmed by a contrast-enhanced abdominal CT performed 40 days after B-RTO. Table 1 shows details of the 6 patients who developed thrombus; none had a history of thrombotic disease. Table 2 shows results of the analysis of the relationships between the development of thrombus in the systemic vein and the investigated variables. In those patients with thrombus development, PT-INR was significantly lower than in the group without thrombus development (p = 0.005). Also, the requirement for the addition of 5% EOI on the second day was significantly lower (p = 0.048) in the group with the development of thrombus.
Among the 49 patients, complications other than the development of thrombus in the inferior vena cava or the right common-external iliac vein included migration of a microcoil used for the embolization of the inferior phrenic vein in 1 patient, stress cardiomyopathy in another, and a thrombus in the splenic vein or the portal vein in three. Decrease in hepatic and renal function was not observed, and none of the patients exhibited symptoms suggestive of pulmonary embolism (including the 6 patients in whom a thrombus developed in the infrarenal inferior vena cava or the right common-external iliac vein).
Balloon-occluded retrograde transvenous obliteration has become widely accepted as a treatment for gastric varices with a gastrorenal shunt (5-17) as its safety and effectiveness were reported by Kanagawa et al. (6). However, there have been many reports of complications of B-RTO, such as hematuria, fever, abdominal pain, pleural effusion, and ascites (5, 7, 10, 11, 13-15). Among severe complications of B-RTO, development of thrombus in the left renal vein, splenic vein, and portal vein were reported by Cho et al. (11) to be 15%. Pulmonary embolism, which was very rare and ranged from 1.5-4.3% (10, 13, 15), was also reported. Shimoda et al. (10) mentioned the cause of pulmonary embolism to be a flow of the sclerosant or fragments of blood clots into the pulmonary artery from a gastrorenal shunt or gastrocaval shunt after balloon deflation. However, we are unaware of reports on the development of thrombosis, in a major systemic vein such as the inferior vena cava and the iliac vein, caused by an indwelled catheter for B-RTO.
Our current analysis showed that, in 6 of 49 patients (12.2%), a thrombus developed in the infrarenal inferior vena cava or right common-external iliac vein. All of these thrombi laid longitudinally on the right side of the inferior vena cava and the right iliac vein, which suggests that the thrombus originated between the B-RTO catheter and the vessel wall. On the other hand, no thrombi developed in the systemic veins into which the B-RTO catheter was not inserted. This suggests that the development of the thrombi was iatrogenic. Although in the majority of these 6 patients, the thrombus was so slight that treatment was unnecessary, the incidence rate of thrombus (12.2%) was relatively high. We should note that most of the patients (37/49 patients) did not undergo a CT scan below the kidney; therefore, the frequency rate of thrombus might have actually been higher than could be determined from our retrospective review of the postprocedural CT scans. Although none of these patients experienced respiratory symptoms, the presence of such a thrombus could lead to pulmonary embolism, which would have clinical implications for the care of such patients.
Our results indicated that a lower PT-INR was a risk factor for the development of thrombosis. Moreover, in patients with thrombus development, the addition of 5% EOI, on the second day, was not frequently needed. Additionally, among our patients, the rate of incomplete thrombosis detected on the second day was relatively higher (35/49 [71%]) than the rate reported in previous works, in which the catheter was retained for fewer than 3 hours; these reported rates ranged from 0% to 45% (5, 7, 8, 10, 12). A potential reason for this high rate might be that we assigned the designation of incomplete thrombosis even if only a tiny part of the gastric varix was stained densely on the retrograde venography. In the original report of B-RTO, by Kanagawa et al. (6), the B-RTO catheter was withdrawn after 30 minutes. However, in more recent reports, the B-RTO catheter was retained overnight in order to avoid incomplete therapeutic efficacy and pulmonary embolism due to an unstable thrombus (13-16). In our study, the duration of indwelling of a catheter had no significant connection to the development of a thrombus. However, as the catheter was left overnight for in patients, differences in duration of indwelling of the catheter were too slight to conclude that duration did not influence thrombus development. Hence, for patients without prolonged coagulation time, retention of a B-RTO catheter for a shorter period than an entire night might be adequate in the efforts to avoid a thrombus in a systemic vein in contact with the B-RTO catheter.
This study had several limitations, such as the small number of cases and nearly identical duration of catheter indwelling between the groups. In the future, studies should be undertaken in order to determine adequate duration of B-RTO catheter retention with a larger number of subjects.
In our study, the frequency of development of a thrombus after B-RTO, in a systemic vein such as the inferior vena cava or the iliac vein, was relatively high. This indicates that physicians should be aware of the possibility of pulmonary embolism caused by a thrombus along the B-RTO catheter in the inferior vena cava or the iliac vein.
Figures and Tables
Fig. 1
58-year-old man with gastric varices. Balloon-occluded retrograde transvenous obliteration (B-RTO) was performed by injecting 20 mL 5% ethanolamine oleate iopamidol (Patient no. 6 in Table 1).
A. Roentgenogram obtained after injection of sclerotic agent showed complete filling by sclerotic agent in gastric varix (arrowhead), curved catheter sheath-introducer inserted to left renal vein (large arrow), and balloon catheter wedged into gastrorenal shunt (small arrow). B. Roentgenogram obtained day following injection of sclerotic agent showed complete thrombosis of varices (arrowhead). Note that sheath-introducer in inferior vena cava is seen (arrows). C-E. Enhanced CT obtained 6 days after B-RTO shows thrombus in infrarenal inferior vena cava and right common-external iliac vein lying longitudinally on right side of vessel wall (arrows).
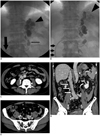
Fig. 2
45-year-old woman with gastric varices. Balloon-occluded retrograde transvenous obliteration (B-RTO) was performed by injecting 15 mL 5% ethanolamine oleate iopamidol (Patient no. 5 in Table 1).
A, B. Enhanced CT obtained 6 days after B-RTO shows thrombus in infrarenal inferior vena cava lying longitudinally on right anterior side of vessel wall (arrows).
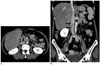
Table 1
Patients with Thrombus Development in Major Systemic Veins after Balloon-Occluded Retrograde Transvenous Obliteration

Table 2
Variables Affecting Thrombus Development in Systemic Vein after Balloon-Occluded Retrograde Transvenous Obliteration

Note.- *p value of < 0.05 was considered to indicate a statistically significant difference, †Data are the mean, ‡Data are the median, §Grade of gastric varices according to Hirota et al. (8). EOI = ethanolamine oleate iopamidol, IVC = inferior vena cava
References
1. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992. 16:1343–1349.
2. Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988. 95:434–440.
3. Pagliaro L, D'Amico G, Luca A, Pasta L, Politi F, Aragona E, et al. Portal hypertension: diagnosis and treatment. J Hepatol. 1995. 23:Suppl 1. 36–44.
4. Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointest Endosc. 1986. 32:264–268.
5. Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001. 12:327–336.
6. Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996. 11:51–58.
7. Koito K, Namieno T, Nagakawa T, Morita K. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996. 167:1317–1320.
8. Hirota S, Matsumoto S, Tomita M, Sako M, Kono M. Retrograde transvenous obliteration of gastric varices. Radiology. 1999. 211:349–356.
9. Sugimori K, Morimoto M, Shirato K, Kokawa A, Tomita N, Numata K, et al. Retrograde transvenous obliteration of gastric varices associated with large collateral veins or a large gastrorenal shunt. J Vasc Interv Radiol. 2005. 16:113–118.
10. Shimoda R, Horiuchi K, Hagiwara S, Suzuki H, Yamazaki Y, Kosone T, et al. Short-term complications of retrograde transvenous obliteration of gastric varices in patients with portal hypertension: effects of obliteration of major portosystemic shunts. Abdom Imaging. 2005. 30:306–313.
11. Cho SK, Shin SW, Do YS, Park KB, Choo SW, Kim SS, et al. Development of thrombus in the major systemic and portal veins after balloon-occluded retrograde transvenous obliteration for treating gastric variceal bleeding: its frequency and outcome evaluation with CT. J Vasc Interv Radiol. 2008. 19:529–538.
12. Katoh K, Sone M, Hirose A, Inoue Y, Fujino Y, Onodera M. Balloon-occluded retrograde transvenous obliteration for gastric varices: the relationship between the clinical outcome and gastrorenal shunt occlusion. BMC Med Imaging. 2010. 10:2.
13. Cho SK, Shin SW, Lee IH, Do YS, Choo SW, Park KB, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol. 2007. 189:W365–W372.
14. Kitamoto M, Imamura M, Kamada K, Aikata H, Kawakami Y, Matsumoto A, et al. Balloon-occluded retrograde transvenous obliteration of gastric fundal varices with hemorrhage. AJR Am J Roentgenol. 2002. 178:1167–1174.
15. Akahoshi T, Hashizume M, Tomikawa M, Kawanaka H, Yamaguchi S, Konishi K, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: a 10-year experience. J Gastroenterol Hepatol. 2008. 23:1702–1709.
16. Ninoi T, Nishida N, Kaminou T, Sakai Y, Kitayama T, Hamuro M, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005. 184:1340–1346.
17. Kiyosue H, Mori H, Matsumoto S, Yamada Y, Hori Y, Okino Y. Transcatheter obliteration of gastric varices: Part 2. Strategy and techniques based on hemodynamic features. Radiographics. 2003. 23:921–937. discussion 937.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download