Abstract
Objective
To assess the technical success and complication rates of the radiologic placement of central venous ports via the internal jugular vein.
Materials and Methods
We retrospectively reviewed 1254 central venous ports implanted at our institution between August 2002 and October 2009. All procedures were guided by using ultrasound and fluoroscopy. Catheter maintenance days, technical success rates, peri-procedural, as well as early and late complication rates were evaluated based on the interventional radiologic reports and patient medical records.
Results
A total of 433386 catheter maintenance days (mean, 350 days; range 0-1165 days) were recorded. The technical success rate was 99.9% and a total of 61 complications occurred (5%), resulting in a post-procedural complication rate of 0.129 of 1000 catheter days. Among them, peri-procedural complications within 24 hours occurred in five patients (0.4%). There were 56 post-procedural complications including 24 (1.9%, 0.055 of 1000 catheter days) early and 32 (2.6%, 0.074 of 1000 catheter days) late complications including, infection (0.6%, 0.018 of 10000 catheter days), thrombotic malfunction (1.4%, 0.040 of 1000 catheter days), nonthrombotic malfunction (0.9%, 0.025 of 1000 catheter days), venous thrombosis (0.5%, 0.014 of 1000 catheter days), as well as wound problems (1.1%, 0.032 of 1000 catheter days). Thirty six CVPs (3%) were removed due to complications. Bloodstream infections and venous thrombosis were the two main adverse events prolonging hospitalization (mean 13 days and 5 days, respectively).
Since the first report of subcutaneous tunneled central venous port (CVP) placement in 1982 (1), a CVP has become widely used in the treatment of oncologic patients undergoing repeated intravenous chemotherapy. Increasing the use of CVPs is attributed to increasing the number of forms of chemotherapy, safe and long-term durability of CVPs, and satisfying quality of life as well as cosmetic results (2-6).
Currently, the radiologic placement of CVPs has become a widely accepted technique. Several studies have demonstrated a high rate of technical success and low rate of complication for radiologic placement, compared to surgical implantations (7-12). In radiologic placement, the internal jugular vein (IJV) is preferred as an access site, as it is safely accessed under ultrasound (US) guidance and located away from the lung or nerve plexus, and results in a low procedural complication rate (10, 11, 13-15).
We began the radiologic placement of CVPs in 2002, and have implanted 1254 CVPs via the IJV in oncologic patients. In this long-term retrospective study, we report a single center experience on a large number of radiologic placements of CVPs exclusively via the IJV, with respect to technical success, complication rates, and clinical outcome.
Between August 2002 and October 2009, 1254 CVPs in 1237 adult patients with an average age of 57 ± 12 years (range 18-84 years) were implanted by an interventional radiology team at our institute. In all patients, CVP placement was indicated for the administration of chemotherapy. We used three kinds of port systems; Celsite (9.6 Fr, B. Braun Medical, Boulogne, France), Vortex (8 Fr, AngioDynamics, Latham, NY, USA), and Healthport (8 Fr, Baxter S.A., Fromet) (Table 1). Exclusion criteria were as follows: active systemic infection, local infection at the port implantation site, uncorrectable coagulopathy with a platelet count < 50/nl, PTT < 50% and INR > 1.5, and acute venous thrombosis or chronic complete obstruction at the IJV or the superior vena cava. Demographic data and underlying diseases are shown in Table 1. Institutional Review Board approval was obtained for this study.
Written informed consent was obtained from each patient before conducting the procedure. The procedure was performed in an interventional radiology suite by 12 interventional radiologists and rotating residents of radiology. It has been our internal policy that residents, after a month of observation, carried out the first 10 implantations under supervision of an experienced interventional radiologist.
Antibiotic prophylaxis was used in high risk patients according to clinicians' decision according to a patient's disease status or absolute neutrophil count. For the patients with absolute neutropenia (white blood cell count < 500/mm3), 1 gram of cefazolin sodium was intravenously administrated before the procedure. None of the procedures employed sedation or was attended by an anesthesiologist.
US examination of the IJV was performed prior to skin preparation in order to screen the patency and the size of the vein, and to rule out venous thrombosis. The right IJV was preferentially selected for the initial access route. However, the left side was chosen in 57 patients due to postmastectomy status, postradiation therapy status, venous thrombosis on surveillance US, or the presence of a previously inserted catheter on the right side.
During the entire procedure, special caution was paid to the maintenance of maximum sterile conditions. After local anesthesia with 1% lidocaine, a selected vessel was punctured under real-time US-guidance using a 21-G needle. After successful puncture, an 0.018-inch wire, followed by a 5 French dilator, was inserted along the wire. The port reservoir pocket was created, and we packed povidone iodine balls within the pocket for hemostasis and disinfection. Then, an 0.035-inch guidewire was advanced through a 5 French dilator to the superior vena cava and further into the inferior vena cava to assure venous puncture. The puncture tract was serially dilated and an 8.5 or 10 French peel-away sheath was inserted into the IJV. The port catheter was tunneled from the pocket to the puncture site and introduced into the superior vena cava through the peel-away sheath. The catheter tip was placed at the level of the cavoatrial junction or 2-3 cm below the carina, and was confirmed by fluoroscopy. In the case where a cavoatrial junction was not conclusive on fluoroscopy, we preferred to place the tip lower rather than higher in relation to the suspected cavoatrial area (16). The patency of the port system was checked by aspirating blood and injecting normal saline without any problem. The skin incision was sutured using nylon 3-0. Suture of the port to the pectoralis fascia was not performed in all implantations. Finally, a fluoroscopic image in the supine position and a chest radiograph in the upright position were obtained to observe the position of the catheter tip and the presence of complications such as pneumothorax.
The CVP was used from the day of implantation or the next. A 21- or 22-G Huber needle was used for infusion of the chemotherapy agent. Before use, a patient's skin was disinfected with povidone iodine and the CVP was tested by aspirating blood and infusing normal saline. After use, normal saline was flushed to fill the port and the catheter. In the condition that the port was not used for more than a month, a diluted heparin solution was flushed every month.
We retrospectively reviewed fluoroscopic images, chest radiographs, and medical records. The deadline of data collection was July 31, 2010. A total of 376 ports reached the deadline. The remaining ports did not reach the deadline due to being lost to follow-up (n = 403), death (n = 127) or removal of CVPs (n = 348) (Fig. 1). The last date of follow-up was defined as the date of the latest visit to the hospital, death date by a death registry on medical records, or the date of CVP removal. Catheter maintenance days were calculated as the number of days between implantation and the last date of follow-up.
Complications were classified into three groups based on the timing of onset; periprocedural (< 24 hours), early (≤ 30 days), or late (> 30 days), which were classified according to the Society of the Interventional Radiology (SIR) Technology guidelines (17).
Catheter dysfunctions encompass any conditions where a CVP is unusable for infusion. These conditions include thrombotic causes (occlusion at the catheter tip or lumen, fibrin-sheath formation, or chamber thrombosis) as well as nonthrombotic causes (catheter migration, kinking of a catheter, or port rotation), which are confirmed by typical imaging findings on fluoroscopic port function check or chest radiographs.
Catheter-related infections are divided into local infections (pocket infection, tunnel infection, or wound infection) and blood stream infections (bacteremia or sepsis). Local infections usually present with erythema, tenderness and/or induration near the pocket or along the tunnel, or are sometimes associated with the presence of pus (5). Catheter-related blood stream infections are defined when a blood culture is positive without other identifiable sources of infection, and clinical signs resolve within 48 hours after port explantation (6). Among them, catheter-related sepsis is specifically defined when clinical signs are compatible with sepsis, which is a systemic inflammatory response syndrome (17).
Complications were graded by its outcome as minor (Group A or B) or major (Group C, D, E, or F) according to SIR classification. Complications requiring hospitalization more than overnight admission were considered as major complications, and major complications were subdivided into four groups as follows: hospitalization less than 48 hours (Group C), prolonged hospitalization of more than 48 hours (Group D), permanent adverse sequelae (Group E) and death (Group F) (17).
A total of 1254 CVPs were implanted in 1237 patients. A total of 433386 catheter maintenance days were recorded, and the mean catheter indwelling time was 350 ± 237 days (range, 1-1165 days). Catheterization was performed via the right IJV in 95.5% of patients, and the left IJV in 4.5% of patients.
During follow-up period, 348 CVPs (27.8%) were explanted. Among these, 307 (24.5%) were removed after completion of chemotherapy or in patients with no further treatment plan, 36 CVPs (2.9%) had to be removed due to complications, and five (0.4%) were explanted upon patients' demands (Fig. 1).
Technical success was achieved in 1253 implantations (technical success rate, 99.9%). In one patient, the right carotid artery was accidentally punctured during a trial of right IJV puncture with a 21-G needle. After needle removal and manual compression, US examination revealed hematoma. We observed five periprocedural complications (0.40%), including the one case of arterial puncture mentioned above. Hematomas developed in three implantations (0.24%), and were initially managed by manual compression and compressive dressings. However, two of them finally had to be explanted because of constant oozing hemorrhage from the pocket and patient discomfort. One patient (0.08%) experienced cardiac arrhythmia during and after the procedure, which was resolved spontaneously (Table 2).
In total, 56 post-procedural complications (4.47%, 0.129/1000 catheter days) occurred over 433386 catheter maintenance days. There were 24 (1.90%, 0.055/1000 catheter days) early and 32 (2.55%, 0.074/1000 catheter days) late complications, which are summarized in Table 3. Late complications developed as late as 418 days after implantation and were attributed to thrombotic catheter occlusion.
A total of 8 infectious complications (0.64%, 0.018/1000 catheter days) occurred. Four infections were early complications, and four were late complications as follows: catheter-related sepsis (n = 1), febrile catheter-related bacteremia (n = 6), and port pocket infection (n = 1). Ports were immediately explanted in all infectious complications, except for one patient of transient febrile bacteremia which was treated with systemic antibiotics.
Catheter dysfunctions occurred in 28 patients (2.24%, 0.065/1000 catheter days); 17 thrombotic (1.36%, 0.040/1000 catheter days) and 11 nonthrombotic (0.88%, 0.025/1000 catheter days) dysfunctions. Thrombotic malfunction tended to develop late, whereas most of nonthrombotic malfunctions were early complications. Of 17 thrombotic dysfunctions including catheter occlusion (n = 5), fibrin sheath formation (n = 9), and chamber thrombosis (n = 3), 10 CVPs had to be explanted. Others were successfully corrected by flushing normal saline (n = 2), thrombolysis using urokinase (n = 2) or endovascular stripping (n = 3). Nonthrombotic dysfunctions included catheter migration (n = 4) (Fig. 2), kinking of the catheter (n = 3), and port rotation (n = 4), which were treated by repositioning (n = 7) or removing (n = 4) in the case of failure of reposition.
We had two Group C complications and 11 Group D complications, which resulted in prolongation of hospitalization (range, 2-31 days). Of the 13 major complications, seven were blood steam infections (mean prolongation of hospitalization, 13.3 days), while six were venous thrombosis (mean prolongation of hospitalization, 6.3 days). These required hospitalization for the administration of intravenous antibiotics and anticoagulants, respectively. There were no serious complications resulting in permanent adverse sequelae or death.
As a reliable and stable central venous access, a CVP has been widely adopted since the 1980s and has become increasingly popular, especially for the care of oncologic patients (11, 18). Several studies have reported CVPs to be more durable and to have a longer patency in terms of lower infectious complication and malfunction rates than tunneled or peripheral catheters (9, 19). In many studies, mean catheter maintenance time was over 10 months (3, 4, 16), which was also shown in our study. Moreover, Gebauer et al. (5) reported a high level of patient satisfaction with CVPs. In spite of the palpable and visible port chamber in the chest wall, it does not limit a patients' daily life and allows for a high quality of life in the patients undergoing long-term intravenous chemotherapy.
Several studies reported that radiologic placement of a CVP is superior to anatomical landmark-guided surgical insertion in terms of technical success, time required for puncture, and complications. The complication rates of the cut-down technique were conducted via the cephalic vein range 16-21% (6, 20, 21). Various complication rates have been reported for surgically implanted CVPs ranging from 5.0-24.6% (6) (4, 7, 8). In the radiologic placement of a CVP, the procedure was performed under the guidance of US and fluoroscopy and using the Seldinger technique (6, 15). US-guidance enables the evaluation of the patency and the size of vessels, clear visualization of a vessel to guide puncture and, therefore, a high technical success rate of nearly 100% (4, 11, 13, 15, 22). Fluoroscopy-guidance enables the visualization of the location of a catheter tip, and the course of a catheter, as well as immediate evaluation of port function. We preferred the IJV for the initial access site than the subclavian vein (SCV). The IJV is considered superior to the SCV technical success, complication rate, and procedure time. Overall technical difficulty and complications reported for SCV access range from 10-20% and 1-30%, respectively (4, 23-25). Even though radiologic guidance may help localization of the SCV and decrease the arterial puncture or pneumothorax, technical difficulties including catheter handling to advance a catheter into the brachiocephalic vein and , in consequence, mal-positioning of a catheter remain due to an acute angle when the SCV joins the IJV to form the brachiocephalic vein (24). In addition, the IJV has a straight course to the superior vena cava, allowing for less contact of the catheter with the vessel wall and thus a lower risk of venous thrombosis (23). Moreover, some complications such as pneumothorax or pinch-off syndrome occur only in SCV access (4, 5, 13, 26).
Total complication rates of radiologic placement of CVPs have reported from 4.4 to 14% in the studies of various population sizes and access routes (3-6, 14, 15, 27, 28). The largest series of 8156 port placements by Moureau et al. (14) reported total complication rate of 0.52 per 1000 catheter days. The latest large study by Teichgräber et al. (6) reported total complication rate of 11.8% in 3160 CVP implantations, and 0.41 complications per 1000 catheter days in 2622 CVP implantations. Our results were comparable to the previous studies when calculating the complication rate by a percentage of total implantations (4.85%), whereas we achieved relatively lower complication rate when calculating it based on the total catheter maintenance days (0.129 per 1000 catheter days) due to relatively longer catheter maintenance days.
Infectious complications are of clinical importance because they are a major cause of hospitalization prolongation, as shown in our study. Various infectious complication rates have been reported in the literature ranging from 1.1 to 8.8%, or from 0.10 to 0.27 per 1000 catheter days (3-6, 13, 14, 29). Moureau et al. (14) reported an infectious complication rate of 0.30 per 1000 catheter days in 8156 CVP implantations. In a systemic review of 18 previous studies including 3007 ports by Maki et al. (30), a blood-stream infection rate of 0.10 per 1000 catheter days was reported. Compared to previous studies, we achieved a lower infectious complication rate (0.64%, 0.018 per 1000 catheter days). We attribute our lower infection rate to our standardized protocol, where we strictly applied aseptic techniques during implantation as well as before or after each use, and we used povidone iodine balls to clear bleeding and eliminate possible causes of infection within the pocket. For now, the benefit of prophylactic antibiotics is controversial (5, 31). In a few studies on risk factor analysis for infectious complications (29, 31), it has been shown that hematogeneous malignancy is better associated with infections than solid malignancies. Therefore, further analysis is warranted considering that a variety of patient factors including age, immune status, underlying disease as well as technical factors such as access site and operators' experience, also might affect the results.
Regarding catheter dysfunction, thrombotic dysfunctions have been reported to be relatively common (3, 5, 6) and have a tendency to occur later than nonthrombotic dysfunction, as also shown in our study. According to the systemic review of 57 articles by Groossens et al. (32), about one-third of previous articles reported catheter malfunction rates less than 1%. However, higher incidences of 9.2% (33) or even 47% (34) were also reported. The differences are considered to arise from the various ways of defining dysfunctions and calculating dysfunction rates. Therefore, Goossens et al. (32) suggested that a well-functioning CVP should be clearly defined, and dysfunction rates should be calculated as a percentage of access attempts to represent more functionality. However, retrospective data acquisition of the number of access attempts seems to be practically neither easy nor reliable.
In managing thrombotic dysfunctions by fibrin sheath formation, we performed endovascular stripping successfully in one-third of cases. However, Heye et al. (35) reported a higher success rate of 91.9% for the endovascular stripping technique when additional techniques to mobilize the distal catheter tip from the vessel wall with a snare catheter were applied.
All dysfunctions due to catheter migration early complications. They were frequently detected on erect chest radiographs in obese women or those with pendulous breasts, as shown in our case (Fig. 2). According to Schutz et al., catheter tips migrated cephalad at an average of 20 mm in the erect position, and tip positioning deep into upper portion of the right atrium decreased the risk of catheter malfunction (16). In addition, catheter malposition increases the risk of venous thrombosis (36), or causes kinking of a retracted cathter (37). Therefore, the placement of the catheter tip deeper into the right atrium in obese patients or those with pendulous breasts is recommended. Rotation of the port is not a frequently reported complication, but we had four cases (0.32%, 0.009 per 1000 catheter days), for which we did not fix with suture. The port base had been fixed to the pectoralis major with suture or glue (18). However, several studies have reported that if the pocket is tight enough, a fixation suture is not necessary (18, 38). If a pocket is created too large or subcutaneous fat tissue is loose, a fixation suture may be helpful to prevent rotation of the port (3).
Our study showed the venous thrombosis to be the second most important complication in terms of prolongation of hospitalization for the use of intravenous anticoagulants (mean hospitalization prolongation: 4.8 days). It has been documented to occur with an incidence of 0.3-11.7% (4, 6, 20, 36) or 0.04-0.105 per 1000 catheter days (5, 6), depending on catheter tip location, catheter insertion site, or indwelling duration (16, 20, 36). We had 6 venous thromboses in the IJV, the superior vena cava, and the right atrium and two of them were associated with catheter malposition. In addition, IJV and right catheterization is associated with a lower risk of venous thrombosis compared to SCV and left catheterization because of the straight course of a catheter and minimized vascular injury by catheter movement (36). However, catheter-related thrombosis did not differ between radiologic placement or the surgical cutdown technique (20), and thromboprophylaxis has no significant effect on the reduction of venous thrombosis (20, 39).
The limitation of our study is, first of all, its retrospective nature. We did not make a statement on complication rates in association with access site, a type of port, or the use of prophylactic antibiotics, because of intrinsic difficulties to control the various relevant factors such as patient primary disease, immune status, age, the number of port usage, port management, and so on. Second, we didn't evaluate subjective symptoms such as pain, vomiting or dizziness because there were no reliable, standardized criteria to score them and clarify the relationship with port implantation. Third, there is a possibility of missing a few complications which were neither stated in medical records nor referred to our intervention team, and immediately managed by clinicians; for instance, tip occlusion which was resolved simply by flushing saline as well as a small amount of hematoma or minor wound dehiscence. This might have resulted in a decrease in the total complication rate, but these complications are not of much clinical importance in terms of outcome or cost.
Our study is a retrospective study of radiologic placement of CVPs, including a large number of study populations at a single institute. This large retrospective study demonstrated that US and fluoroscopy-guided CVP placement, especially via the IJV, is safe and effective with an extremely high technical success rate, low peri- and post-procedural complication rates, and satisfactory clinical outcome.
Figures and Tables
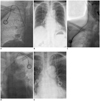
Fig. 2
Case of catheter migration.
A. We placed central venous port via right internal jugular vein in 68 year-old woman with its tip just below carina at T5 level. B. Chest radiograph in erect position showed that catheter is retracted and tip is located somewhere in brachiocephalic or internal jugular vein. Patient is obese with pendulous breasts. C. On fluoroscopy in supine position, catheter tip is further migrated cephalad into right internal jugular vein. Patient complained of neck pain on infusion. D. After removal of port, another port was implanted via left internal jugular vein, and tip was placed deeper in upper portion of right atrium. E. Chest radiograph in erect position revealed that catheter was once again retracted with its tip probably located in left brachiocephalic vein.
References
1. Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982. 92:706–712.
2. Biffi R, de Braud F, Orsi F, Pozzi S, Mauri S, Goldhirsch A, et al. Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann Oncol. 1998. 9:767–777.
3. Cil BE, Canyiğit M, Peynircioğlu B, Hazirolan T, Carkaci S, Cekirge S, et al. Subcutaneous venous port implantation in adult patients: a single center experience. Diagn Interv Radiol. 2006. 12:93–98.
4. Dede D, Akmangit I, Yildirim ZN, Sanverdi E, Sayin B. Ultrasonography and fluoroscopy-guided insertion of chest ports. Eur J Surg Oncol. 2008. 34:1340–1343.
5. Gebauer B, El-Sheik M, Vogt M, Wagner HJ. Combined ultrasound and fluoroscopy guided port catheter implantation--high success and low complication rate. Eur J Radiol. 2009. 69:517–522.
6. Teichgräber UK, Kausche S, Nagel SN, Gebauer B. Outcome analysis in 3,160 implantations of radiologically guided placements of totally implantable central venous port systems. Eur Radiol. 2011. 21:1224–1232.
7. Araújo C, Silva JP, Antunes P, Fernandes JM, Dias C, Pereira H, et al. A comparative study between two central veins for the introduction of totally implantable venous access devices in 1201 cancer patients. Eur J Surg Oncol. 2008. 34:222–226.
8. Biffi R, Pozzi S, Agazzi A, Pace U, Floridi A, Cenciarelli S, et al. Use of totally implantable central venous access ports for high-dose chemotherapy and peripheral blood stem cell transplantation: results of a monocentre series of 376 patients. Ann Oncol. 2004. 15:296–300.
9. Karakousis CP. Surgical technique for totally implantable access ports (TIAP) needs improvement. J Surg Oncol. 2007. 95:180–181.
10. Koroglu M, Demir M, Koroglu BK, Sezer MT, Akhan O, Yildiz H, et al. Percutaneous placement of central venous catheters: comparing the anatomical landmark method with the radiologically guided technique for central venous catheterization through the internal jugular vein in emergent hemodialysis patients. Acta Radiol. 2006. 47:43–47.
11. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996. 24:2053–2058.
12. Seiler CM, Frohlich BE, Dorsam UJ, Kienle P, Buchler MW, Knaebel HP. Surgical technique for totally implantable access ports (TIAP) needs improvement: a multivariate analysis of 400 patients. J Surg Oncol. 2006. 93:24–29.
13. Lorch H, Zwaan M, Kagel C, Weiss HD. Central venous access ports placed by interventional radiologists: experience with 125 consecutive patients. Cardiovasc Intervent Radiol. 2001. 24:180–184.
14. Moureau N, Poole S, Murdock MA, Gray SM, Semba CP. Central venous catheters in home infusion care: outcomes analysis in 50,470 patients. J Vasc Interv Radiol. 2002. 13:1009–1016.
15. Yip D, Funaki B. Subcutaneous chest ports via the internal jugular vein. A retrospective study of 117 oncology patients. Acta Radiol. 2002. 43:371–375.
16. Schutz JC, Patel AA, Clark TW, Solomon JA, Freiman DB, Tuite CM, et al. Relationship between chest port catheter tip position and port malfunction after interventional radiologic placement. J Vasc Interv Radiol. 2004. 15:581–587.
17. Silberzweig JE, Sacks D, Khorsandi AS, Bakal CW;. Reporting standards for central venous access. J Vasc Interv Radiol. 2003. 14:S443–S452.
18. Jan HC, Chou SJ, Chen TH, Lee CI, Chen TK, Lou MA. Management and prevention of complications of subcutaneous intravenous infusion port. Surg Oncol. 2010. [Epub ahead of print].
19. Funaki B. Central venous access: a primer for the diagnostic radiologist. AJR Am J Roentgenol. 2002. 179:309–318.
20. Biffi R, Orsi F, Pozzi S, Pace U, Bonomo G, Monfardini L, et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol. 2009. 20:935–940.
21. Chen PT, Sung CS, Wang CC, Chan KH, Chang WK, Hsu WH. Experience of anesthesiologists with percutaneous nonangiographic venous access. J Clin Anesth. 2007. 19:609–615.
22. LaBella G, Kerlakian G, Muck P, Chung D, Vaughan A, Ritchison A. Port-A-Cath placement without the aid of fluoroscopy or localizing devices: a community hospital series. Cancer J. 2005. 11:157–159.
23. Kurul S, Saip P, Aydin T. Totally implantable venous-access ports: local problems and extravasation injury. Lancet Oncol. 2002. 3:684–692.
24. Kusminsky RE. Complications of central venous catheterization. J Am Coll Surg. 2007. 204:681–696.
25. Lefrant JY, Cuvillon P, Bénézet JF, Dauzat M, Peray P, Saïssi G, et al. Pulsed Doppler ultrasonography guidance for catheterization of the subclavian vein: a randomized study. Anesthesiology. 1998. 88:1195–1201.
26. Hinke DH, Zandt-Stastny DA, Goodman LR, Quebbeman EJ, Krzywda EA, Andris DA. Pinch-off syndrome: a complication of implantable subclavian venous access devices. Radiology. 1990. 177:353–356.
27. Funaki B, Szymski GX, Hackworth CA, Rosenblum JD, Burke R, Chang T, et al. Radiologic placement of subcutaneous infusion chest ports for long-term central venous access. AJR Am J Roentgenol. 1997. 169:1431–1434.
28. Sticca RP, Dewing BD, Harris JD. Outcomes of surgical and radiologic placed implantable central venous access ports. Am J Surg. 2009. 198:829–833.
29. Hsieh CC, Weng HH, Huang WS, Wang WK, Kao CL, Lu MS, et al. Analysis of risk factors for central venous port failure in cancer patients. World J Gastroenterol. 2009. 15:4709–4714.
30. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006. 81:1159–1171.
31. Samaras P, Dold S, Braun J, Kestenholz P, Breitenstein S, Imhof A, et al. Infectious port complications are more frequent in younger patients with hematologic malignancies than in solid tumor patients. Oncology. 2008. 74:237–244.
32. Goossens GA, Stas M, Jérôme M, Moons P. Systematic review: malfunction of totally implantable venous access devices in cancer patients. Support Care Cancer. 2011. 19:883–898.
33. Vandoni RE, Guerra A, Sanna P, Bogen M, Cavalli F, Gertsch P. Randomised comparison of complications from three different permanent central venous access systems. Swiss Med Wkly. 2009. 139:313–316.
34. Frank JL, Garb JL, Halla B, Reed WP Jr. Ionic implantation of silicone chronic venous access devices does not alter thrombotic complications: a double-blinded, randomized clinical trial. Surgery. 2001. 129:547–551.
35. Heye S, Maleux G, Goossens GA, Vaninbroukx J, Jerôme M, Stas M. Feasibility and Safety of Endovascular Stripping of Totally Implantable Venous Access Devices. Cardiovasc Intervent Radiol. 2011. [Epub ahead of print].
36. Luciani A, Clement O, Halimi P, Goudot D, Portier F, Bassot V, et al. Catheter-related upper extremity deep venous thrombosis in cancer patients: a prospective study based on Doppler US. Radiology. 2001. 220:655–660.
37. Shin BS, Ahn M. Implantable central venous chemoport: comparision of results according to approach routes and methods. J Korean Radiol Soc. 2003. 49:165–171.
38. Kim SS, Kim HP, Bae JI, Won JH. Evaluation of the necessity of port fixation in central venous port implantation. J Korean Soc Radiol. 2010. 63:217–220.
39. Akl EA, Kamath G, Yosuico V, Kim SY, Barba M, Sperati F, et al. Thromboprophylaxis for patients with cancer and central venous catheters: a systematic review and a meta-analysis. Cancer. 2008. 112:2483–2492.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download